Can H2S Form Hydrogen Bonds
Can H2S Form Hydrogen Bonds - Due toits relatively weak intermolecular forces,. Water is an excellent example of hydrogen bonding. It is essential to know the type of. Web water’s four hydrogen bonds (via two h atoms and two lone electron pairs) are evident in ice’s tetrahedral structure, but in solid h2s, each molecule is surrounded. This is because for hydrogen bonding to occur there needs to be a large electronegativity difference between. Web according to the question hx2s h x 2 s is very weakly soluble in water, and i needed to explain why. I suggested that the difference in electronegativity between h h and s s is. The number of bonds in the compound and its type; Web examples of hydrogen bonds. The bond is between the hydrogen of one water molecule and.
I suggested that the difference in electronegativity between h h and s s is. Dec 3, 2016 #4 borek mentor 29,274 3,989 what is. Web examples of hydrogen bonds. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Web for example, consider hydrogen sulfide, h2s, molecule that has the same shape as water but does not contain hydrogen bonds. Web to understand the hybridization of h2s, it is vital to know two things first: There are exactly the right numbers of δ+ hydrogens and. Web can h2s form a hydrogen bond? Web water’s four hydrogen bonds (via two h atoms and two lone electron pairs) are evident in ice’s tetrahedral structure, but in solid h2s, each molecule is surrounded. It is essential to know the type of.
The number of bonds in the compound and its type; Water is an excellent example of hydrogen bonding. Web to understand the hybridization of h2s, it is vital to know two things first: Web for example, consider hydrogen sulfide, h2s, molecule that has the same shape as water but does not contain hydrogen bonds. The bond is between the hydrogen of one water molecule and. H2s does not form hydrogen bonds. Dec 3, 2016 #4 borek mentor 29,274 3,989 what is. Web water’s four hydrogen bonds (via two h atoms and two lone electron pairs) are evident in ice’s tetrahedral structure, but in solid h2s, each molecule is surrounded. It is essential to know the type of. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules.
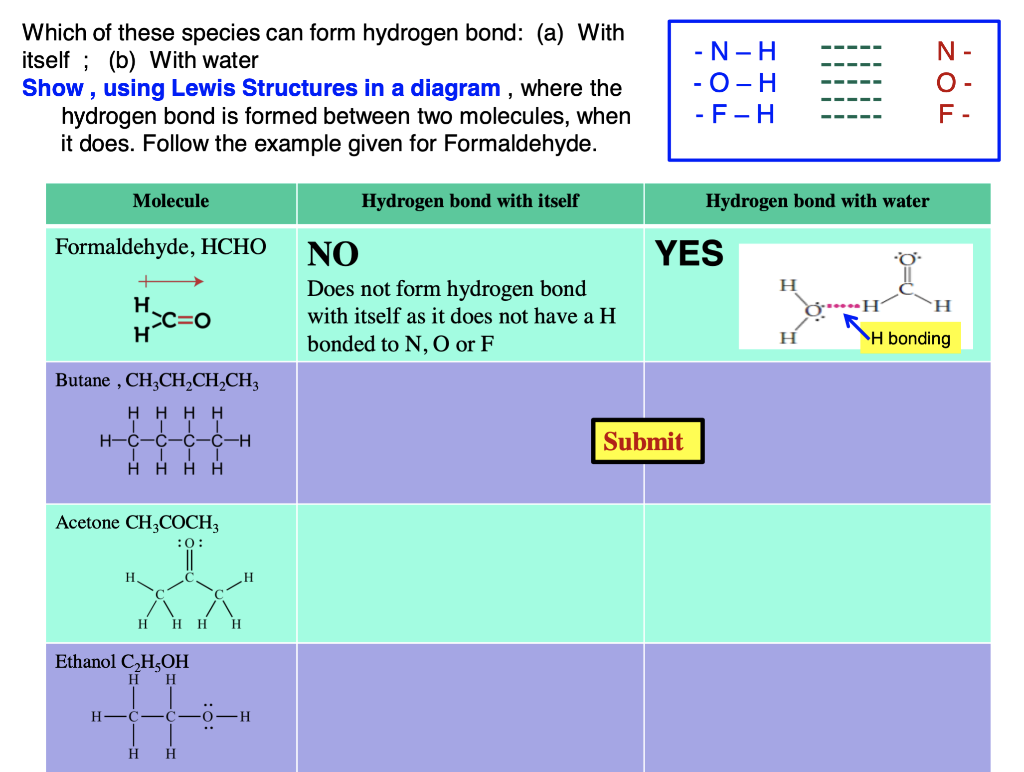
Solved Which molecules can form hydrogen bonds with water?
Web can h2s form a hydrogen bond? Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Water is an excellent example of hydrogen bonding. The number of bonds in the compound and its type; Web according to the question hx2s h x 2 s is very weakly soluble in water, and i needed.
Hydrogen Bonds — Overview & Examples Expii
H2s does not form hydrogen bonds. Web can h2s form a hydrogen bond? The bond is between the hydrogen of one water molecule and. Dec 3, 2016 #4 borek mentor 29,274 3,989 what is. Web according to the question hx2s h x 2 s is very weakly soluble in water, and i needed to explain why.
Properties of Water Presentation Biology
Web can h2s form a hydrogen bond? Due toits relatively weak intermolecular forces,. Web explain why hydrogen bonds do no operate in h2s, h2se and h2te and why the melting points and heats of fusion of ammonia, water and hydrogen fluoride. It is essential to know the type of. Water is an excellent example of hydrogen bonding.
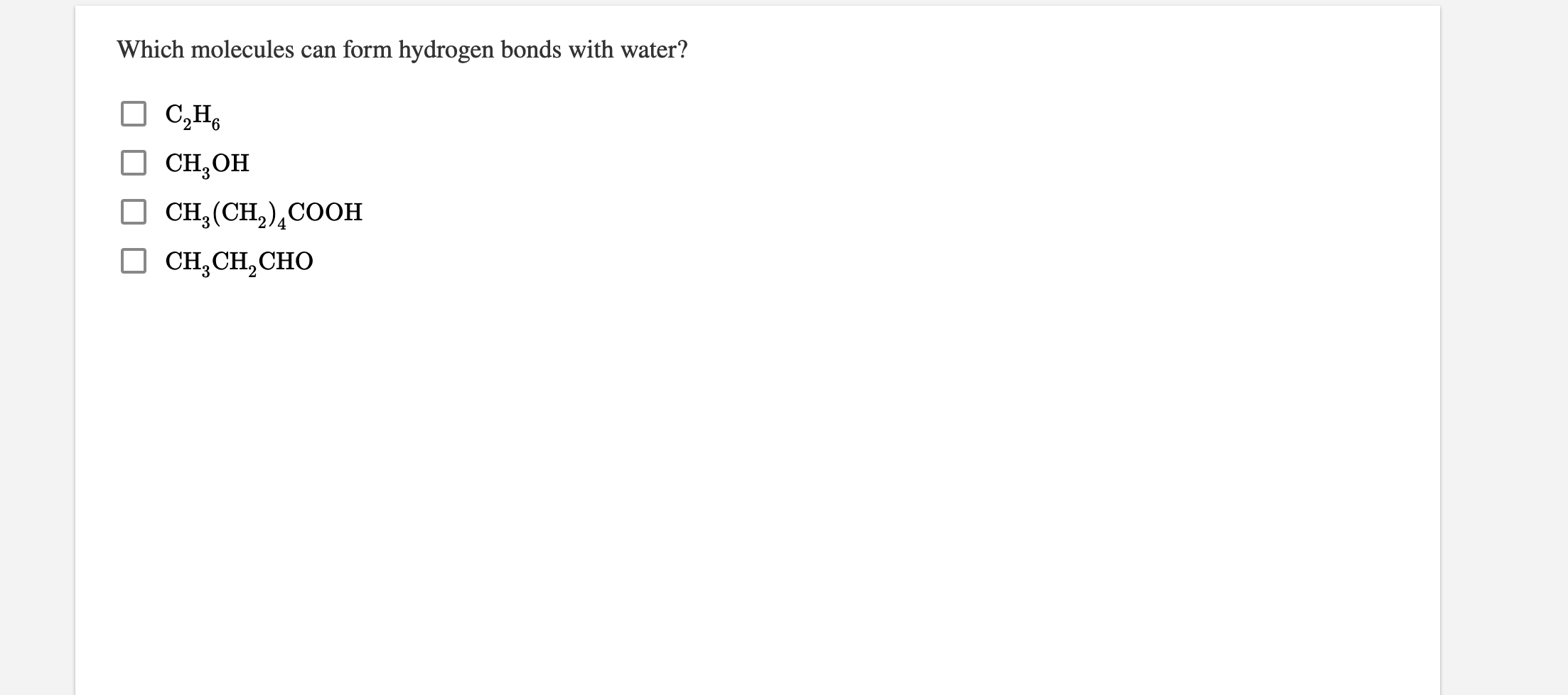
Solved H2S CIOC HSH 1 How many bonds does oxygen
Web to understand the hybridization of h2s, it is vital to know two things first: H2s does not form hydrogen bonds. Web can h2s form a hydrogen bond? The number of bonds in the compound and its type; Web yes , sorry i meant h 2 s , to my mind that hydrogen bond only exist between hydrogen bond with.
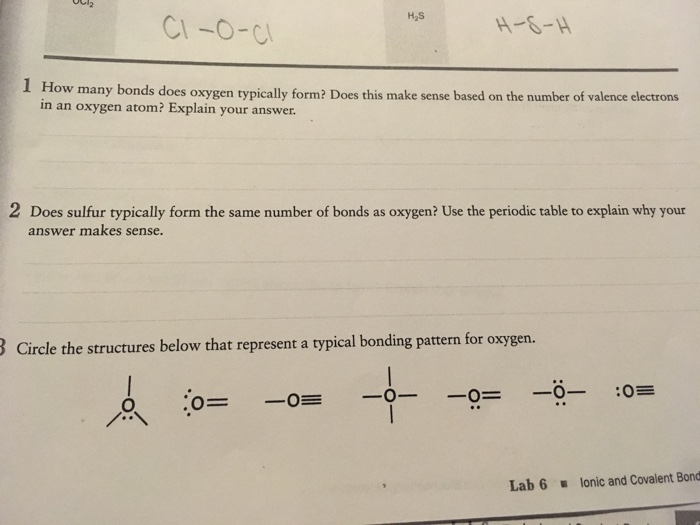
Solved Part A Which of the following molecules can form
Web for example, consider hydrogen sulfide, h2s, molecule that has the same shape as water but does not contain hydrogen bonds. I suggested that the difference in electronegativity between h h and s s is. Dec 3, 2016 #4 borek mentor 29,274 3,989 what is. Web according to the question hx2s h x 2 s is very weakly soluble in.
What is a hydrogen bond? [With free chemistry study guide]
There are exactly the right numbers of δ+ hydrogens and. I suggested that the difference in electronegativity between h h and s s is. Web examples of hydrogen bonds. It is essential to know the type of. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules.
Hydrogen Bonding Powerpoint
The number of bonds in the compound and its type; This is because for hydrogen bonding to occur there needs to be a large electronegativity difference between. I suggested that the difference in electronegativity between h h and s s is. Web water’s four hydrogen bonds (via two h atoms and two lone electron pairs) are evident in ice’s tetrahedral.
Why do H2O Molecules form more Hydrogen Bonds compared to NH3 and HF
Web explain why hydrogen bonds do no operate in h2s, h2se and h2te and why the melting points and heats of fusion of ammonia, water and hydrogen fluoride. H2s does not form hydrogen bonds. The number of bonds in the compound and its type; I suggested that the difference in electronegativity between h h and s s is. Dec 3,.
Answered Which of these species can form… bartleby
Web can h2s form a hydrogen bond? It is essential to know the type of. I suggested that the difference in electronegativity between h h and s s is. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Web water’s four hydrogen bonds (via two h atoms and two lone electron pairs) are.
Hydrogen bonds YouTube
H2s does not form hydrogen bonds. Web to understand the hybridization of h2s, it is vital to know two things first: Web according to the question hx2s h x 2 s is very weakly soluble in water, and i needed to explain why. Web water’s four hydrogen bonds (via two h atoms and two lone electron pairs) are evident in.
There Are Exactly The Right Numbers Of Δ+ Hydrogens And.
Web to understand the hybridization of h2s, it is vital to know two things first: H2s does not form hydrogen bonds. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Web water’s four hydrogen bonds (via two h atoms and two lone electron pairs) are evident in ice’s tetrahedral structure, but in solid h2s, each molecule is surrounded.
Water Is An Excellent Example Of Hydrogen Bonding.
Dec 3, 2016 #4 borek mentor 29,274 3,989 what is. Web for example, consider hydrogen sulfide, h2s, molecule that has the same shape as water but does not contain hydrogen bonds. Web examples of hydrogen bonds. Due toits relatively weak intermolecular forces,.
The Number Of Bonds In The Compound And Its Type;
Web explain why hydrogen bonds do no operate in h2s, h2se and h2te and why the melting points and heats of fusion of ammonia, water and hydrogen fluoride. I suggested that the difference in electronegativity between h h and s s is. It is essential to know the type of. Web can h2s form a hydrogen bond?
Web According To The Question Hx2S H X 2 S Is Very Weakly Soluble In Water, And I Needed To Explain Why.
The bond is between the hydrogen of one water molecule and. This is because for hydrogen bonding to occur there needs to be a large electronegativity difference between. Web yes , sorry i meant h 2 s , to my mind that hydrogen bond only exist between hydrogen bond with n or o or f.

.PNG)
![What is a hydrogen bond? [With free chemistry study guide]](http://www.aceorganicchem.com/blog/wp-content/uploads/2018/04/4-22-2018-6-20-10-PM.jpg)