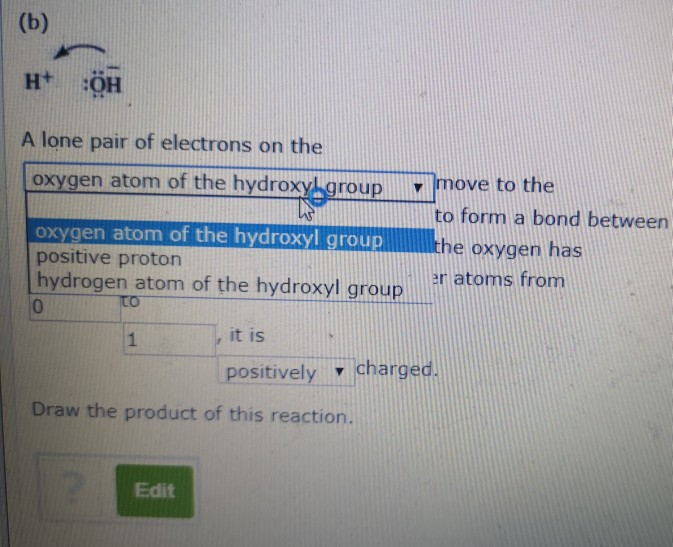
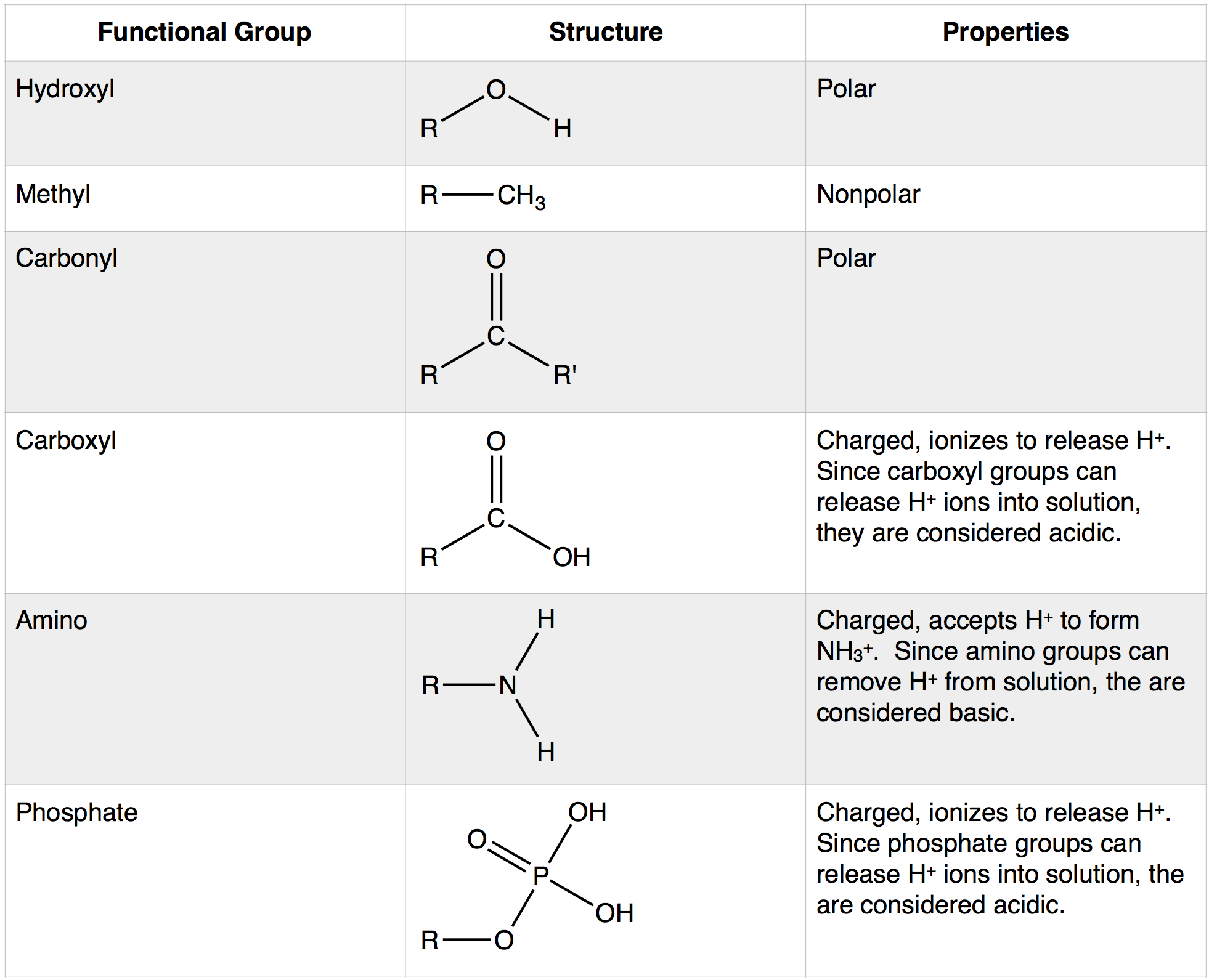
Can Hydroxyl Groups Form Hydrogen Bonds
Can Hydroxyl Groups Form Hydrogen Bonds - Note that hydrogen atom polar in the alcohol shown. Without the strongly polarized o―h bond, ether molecules cannot engage in hydrogen bonding with each other. Any lone electron pairs present on the oxygen or nitrogen in. Web the hydroxyl groups form conjugated hydrogen bonds in separate domains. Web ethers lack the hydroxyl groups of alcohols. They readily participate in hydrogen bonding, generating. The sugars glucose and sucrose are. Due to the high electronegativity of the oxygen atom, the bond between. Web a hydroxyl group (oh group) consists of an oxygen atom covalently bonded to a hydrogen atom. Due to the high electronegativity of the oxygen atom, the bond.
Web in chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula −oh and composed of one oxygen atom covalently bonded to one hydrogen atom. Due to the high electronegativity of the oxygen atom, the bond between. Web two amino acids, serine and threonine, contain aliphatic hydroxyl groups (that is, an oxygen atom bonded to a hydrogen atom, represented as ―oh). The hydroxyl group (oh group) can form hydrogen bonds with water as shown below. Due to the high electronegativity of the oxygen atom, the bond. Any lone electron pairs present on the oxygen or nitrogen in. Note that hydrogen atom polar in the alcohol shown. The sugars glucose and sucrose are. The ab initio molecular dynamics gave us a possibility to understand the. Without the strongly polarized o―h bond, ether molecules cannot engage in hydrogen bonding with each other.
Note that hydrogen atom polar in the alcohol shown. Any lone electron pairs present on the oxygen or nitrogen in. Due to the high electronegativity of the oxygen atom, the bond between. The sugars glucose and sucrose are. Web two amino acids, serine and threonine, contain aliphatic hydroxyl groups (that is, an oxygen atom bonded to a hydrogen atom, represented as ―oh). Web in this review we present a comprehensive examination of the scientific literature in the area, with focus on theory and molecular simulation, and conclude that. Without the strongly polarized o―h bond, ether molecules cannot engage in hydrogen bonding with each other. Web the hydrogen bond is also responsible for the existence as solids of many organic molecules containing hydroxyl groups (―oh); Web in chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula −oh and composed of one oxygen atom covalently bonded to one hydrogen atom. Web a hydroxyl group (oh group) consists of an oxygen atom covalently bonded to a hydrogen atom.
Classes of Organic Compounds Boundless Chemistry
Web in this review we present a comprehensive examination of the scientific literature in the area, with focus on theory and molecular simulation, and conclude that. Due to the high electronegativity of the oxygen atom, the bond. The ab initio molecular dynamics gave us a possibility to understand the. Note that hydrogen atom polar in the alcohol shown. Web the.
Tetryonics 102.06 HydrogenOxygen [OH] bonds form the hydroxyl group
Web two amino acids, serine and threonine, contain aliphatic hydroxyl groups (that is, an oxygen atom bonded to a hydrogen atom, represented as ―oh). Note that hydrogen atom polar in the alcohol shown. Without the strongly polarized o―h bond, ether molecules cannot engage in hydrogen bonding with each other. The ab initio molecular dynamics gave us a possibility to understand.
Intro Bio Lec. 2 Columbia University
Web hydroxyl groups are simple structures consisting of an oxygen atom with two lone pairs bonded to a hydrogen atom. Web ethers lack the hydroxyl groups of alcohols. Web in chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula −oh and composed of one oxygen atom covalently bonded to one hydrogen atom. The ab initio.
Figure 3.15. Hydrogen Bonding in Water
The ab initio molecular dynamics gave us a possibility to understand the. Web hydroxyl groups are simple structures consisting of an oxygen atom with two lone pairs bonded to a hydrogen atom. Web in chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula −oh and composed of one oxygen atom covalently bonded to one hydrogen.
What Is the OH Functional Group Called?
They readily participate in hydrogen bonding, generating. Web in chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula −oh and composed of one oxygen atom covalently bonded to one hydrogen atom. Web a hydroxyl group (oh group) consists of an oxygen atom covalently bonded to a hydrogen atom. Web hydroxyl groups are simple structures consisting.
Illustration diagram of hydrogen bonding between hydroxyl and NH 3 (OH⋅
The hydroxyl group (oh group) can form hydrogen bonds with water as shown below. Due to the high electronegativity of the oxygen atom, the bond between. Web the hydrogen bond is also responsible for the existence as solids of many organic molecules containing hydroxyl groups (―oh); Web in chemistry, a hydroxy or hydroxyl group is a functional group with the.
Xaa 3(S)Hyp intermolecular hydroxyl hydrogen bonds. Shown are the two
The sugars glucose and sucrose are. Web the hydrogen bond is also responsible for the existence as solids of many organic molecules containing hydroxyl groups (―oh); Web the hydroxyl groups form conjugated hydrogen bonds in separate domains. Web two amino acids, serine and threonine, contain aliphatic hydroxyl groups (that is, an oxygen atom bonded to a hydrogen atom, represented as.
His124 forms a hydrogen bond with a hydroxyl group of gallate and
Web two amino acids, serine and threonine, contain aliphatic hydroxyl groups (that is, an oxygen atom bonded to a hydrogen atom, represented as ―oh). Due to the high electronegativity of the oxygen atom, the bond between. Any lone electron pairs present on the oxygen or nitrogen in. They readily participate in hydrogen bonding, generating. Web a hydroxyl group (oh group).
Solved Chapter 11, Question 18 For each of the following
Web hydroxyl groups are simple structures consisting of an oxygen atom with two lone pairs bonded to a hydrogen atom. Without the strongly polarized o―h bond, ether molecules cannot engage in hydrogen bonding with each other. Web in chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula −oh and composed of one oxygen atom covalently.
S2019_Lecture_02_Reading Biology LibreTexts
Due to the high electronegativity of the oxygen atom, the bond. Web a hydroxyl group (oh group) consists of an oxygen atom covalently bonded to a hydrogen atom. Note that hydrogen atom polar in the alcohol shown. Web the hydrogen bond is also responsible for the existence as solids of many organic molecules containing hydroxyl groups (―oh); The hydroxyl group.
They Readily Participate In Hydrogen Bonding, Generating.
Web a hydroxyl group (oh group) consists of an oxygen atom covalently bonded to a hydrogen atom. Web in this review we present a comprehensive examination of the scientific literature in the area, with focus on theory and molecular simulation, and conclude that. Web the hydrogen bond is also responsible for the existence as solids of many organic molecules containing hydroxyl groups (―oh); Without the strongly polarized o―h bond, ether molecules cannot engage in hydrogen bonding with each other.
The Hydroxyl Group (Oh Group) Can Form Hydrogen Bonds With Water As Shown Below.
Due to the high electronegativity of the oxygen atom, the bond. Web in chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula −oh and composed of one oxygen atom covalently bonded to one hydrogen atom. Web two amino acids, serine and threonine, contain aliphatic hydroxyl groups (that is, an oxygen atom bonded to a hydrogen atom, represented as ―oh). Web hydroxyl groups are simple structures consisting of an oxygen atom with two lone pairs bonded to a hydrogen atom.
Web The Hydroxyl Groups Form Conjugated Hydrogen Bonds In Separate Domains.
The ab initio molecular dynamics gave us a possibility to understand the. Web ethers lack the hydroxyl groups of alcohols. Any lone electron pairs present on the oxygen or nitrogen in. The sugars glucose and sucrose are.
Due To The High Electronegativity Of The Oxygen Atom, The Bond Between.
Note that hydrogen atom polar in the alcohol shown. Web a hydroxyl group (oh group) consists of an oxygen atom covalently bonded to a hydrogen atom.

![Tetryonics 102.06 HydrogenOxygen [OH] bonds form the hydroxyl group](https://i.pinimg.com/originals/2f/13/da/2f13dad7bddde5e7b4d3143c0802ada5.jpg)
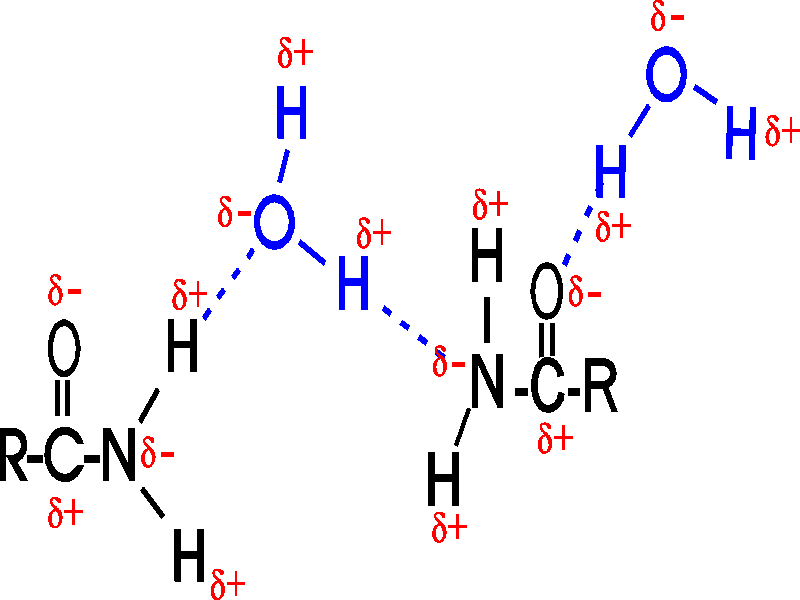
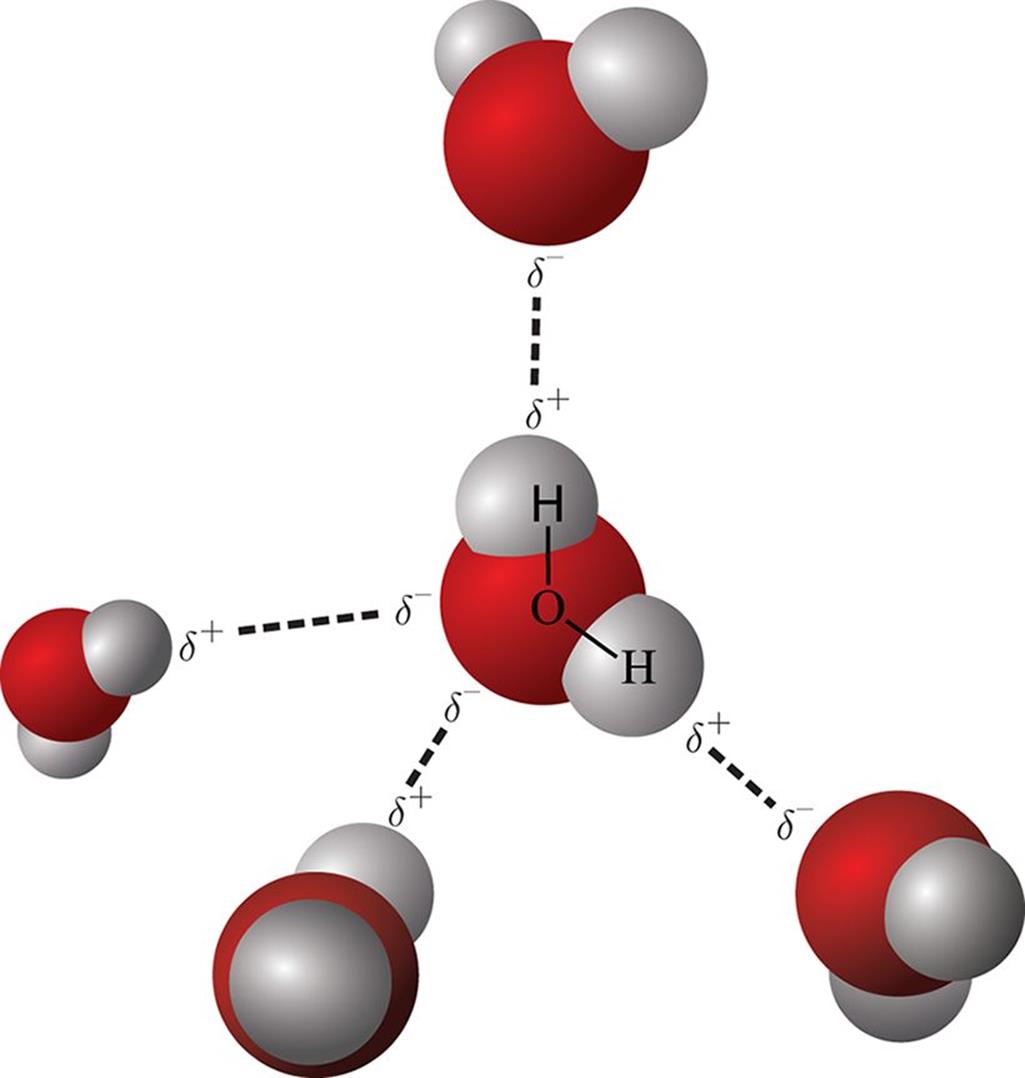
:max_bytes(150000):strip_icc()/hydroxylfunctional-56a129e05f9b58b7d0bca5df.jpg)