Carbon Dioxide Dissolves In Water To Form
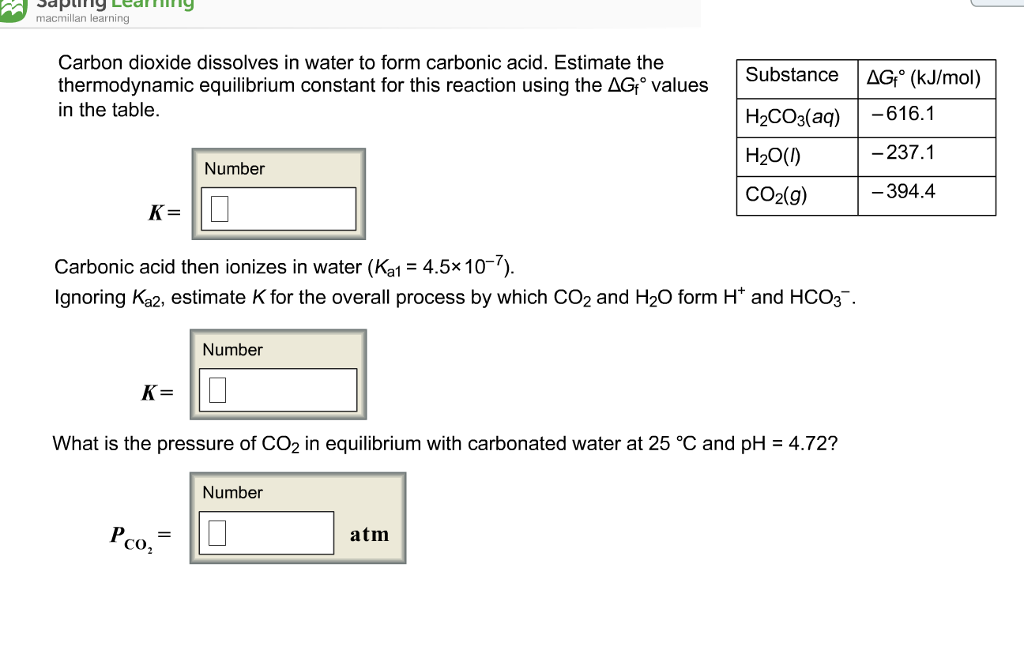
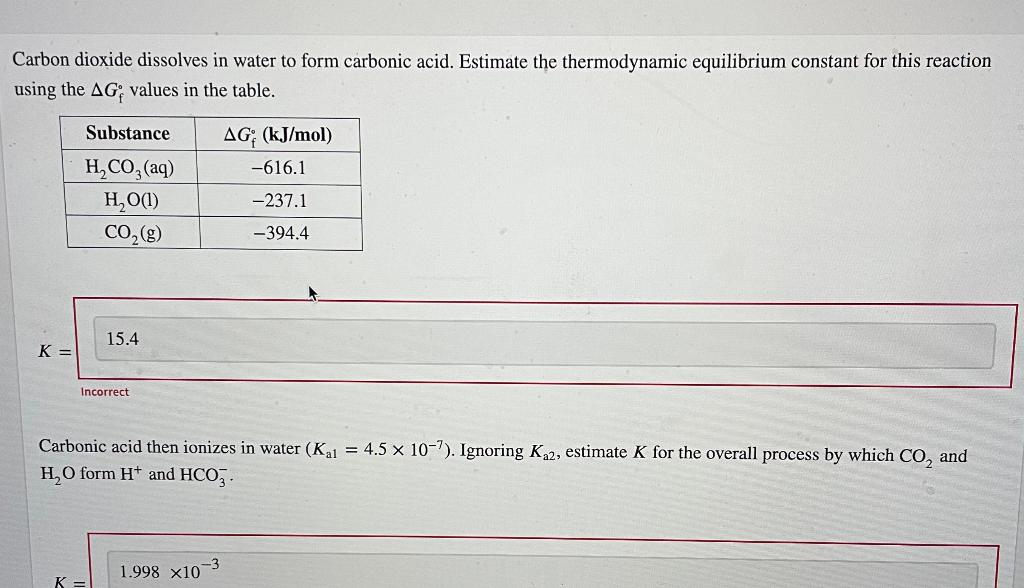
Carbon Dioxide Dissolves In Water To Form - Carbon dioxide dissolves in water to form carbonic acid. Most of it gets dissolved. And the rate at which carbon dioxide is removed from the air depends partly on the activities of water plants. Web in reaction , gaseous carbon dioxide is dissolved in water, reacting to form carbonic acid. Most of it gets dissolved, co 2 remains loosely hydrated. Holding 100ml of water (ebkare)________________2. Estimate the thermodynamic equilibrium constant for this reaction using the ag, values in the table. Carbon dioxide is also an acid anhydride because on reacting with water it. Vapour pressure of water is negligible. Web answer (1 of 2):
Web answer (1 of 2): Web chemistry chemistry questions and answers carbon dioxide dissolves in water to form carbonic acid. Estimate the thermodynamic equilibrium constant for this reaction using the ag, values in the table. Dissolving anything is a physical change. Hydrogen ions dissociate from the carbonic acid, to give bicarbonate (. Web the solubility of carbon dioxide in water decreases as the temperature is raised, and it is driven off into the atmosphere. Co2 dissolves in water to form carbonic acid: Vapour pressure of water is negligible. Web cold water can dissolve more carbon dioxide than warm water. Estimate the thermodynamic equilibrium constant for this reaction using.
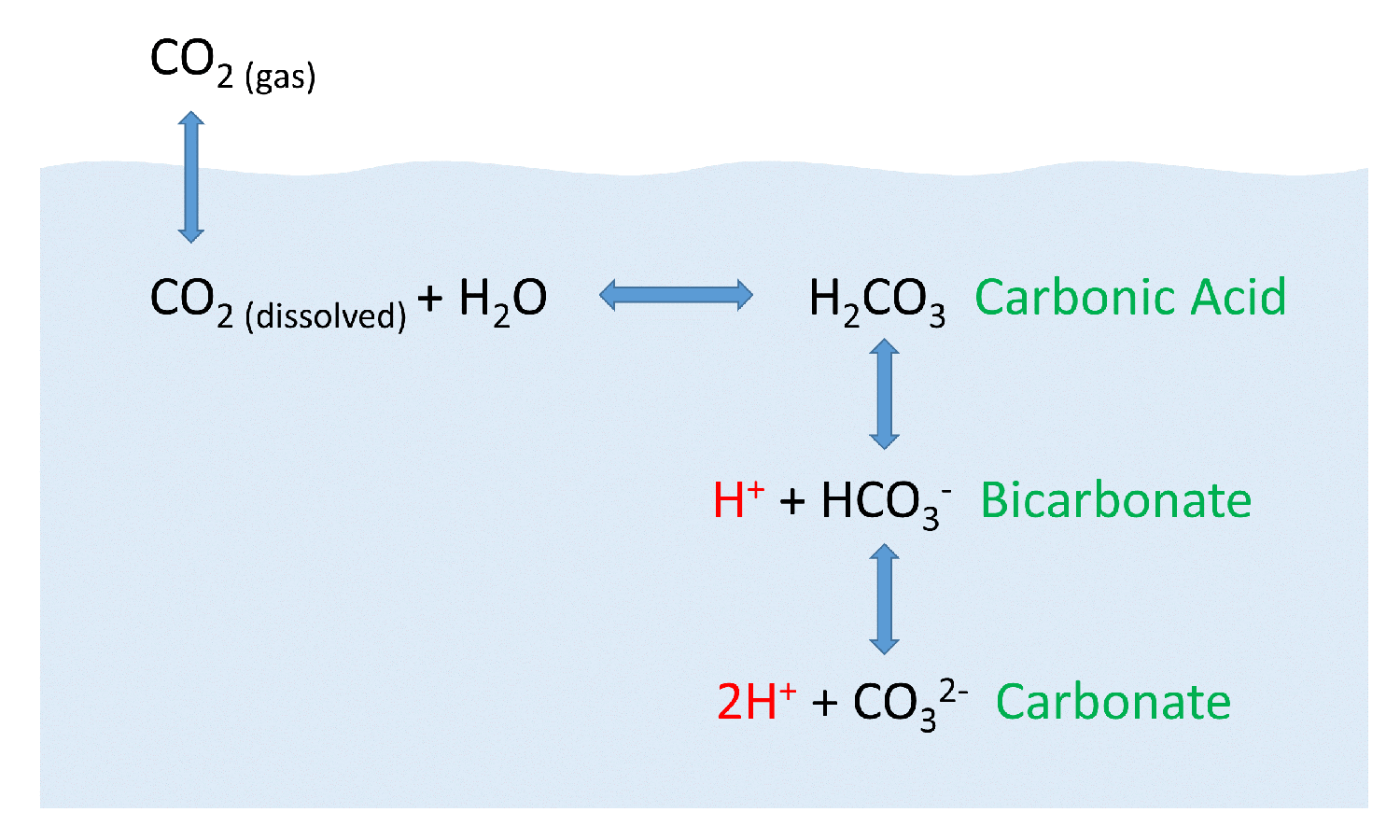
Web carbon dioxide dissolves in water to form carbonic acid. Web when carbon dioxide dissolves in water only some molecules of it will react with water. Web carbon dioxide is soluble in water, in which it reversibly forms. H 2co 3 is a. Holding 100ml of water (ebkare)________________2. The bicarbonate ions can further. Web co2 is quite soluble in water: (carbonic acid), which is a weak acid since its ionization in water is incomplete. Web chemistry chemistry questions and answers carbon dioxide dissolves in water to form carbonic acid. Dissolved co, satisfies the equilibrium.
Water Analysis Dissolved Carbon Dioxide Advanced BioTech
Web carbon dioxide is soluble in water, in which it reversibly forms. Estimate the thermodynamic equilibrium constant for this reaction using. Estimate the thermodynamic equilibrium constanst (k) for this reaction (delta gf values: Estimate the thermodynamic equilibrium constant for this reaction using the ag, values in the table. Web answer (1 of 2):
Summary of the reactions between carbon dioxide (CO2) with water (H2O
Co2 dissolves in water to form carbonic acid: Web co2 is quite soluble in water: Volume of solution does not change on dissolution of. The concentration of dissolved carbon dioxide therefore. Estimate the thermodynamic equilibrium constant for this reaction using.
Transforming Carbon Dioxide to Into Fuel More Efficiently With a Water
Web correct option is a) when co 2 dissolves in h 2o, only some molecules react with h 2) to form h 2co 3. Web when carbon dioxide dissolves in water only some molecules of it will react with water. Dissolved carbon dioxide is in chemical equilibrium with carbonic acid, which in turn is in equilibrium with bicarbonate. Dissolving anything.
😂 When carbon dioxide dissolves in water. What does carbon dioxide form
Hydrogen ions dissociate from the carbonic acid, to give bicarbonate (. Co2 dissolves in water to form carbonic acid: 30% of all carbon dioxide is dissolved in water. Estimate the thermodynamic equilibrium constant for this reaction using. It reacts with water ( h 2 o) and forms carbonic acid ( h 2.
Understanding the Science of Ocean and Coastal Acidification Ocean
Web carbon dioxide is soluble in water, in which it reversibly forms. The bicarbonate ions can further. Dissolved carbon dioxide is in chemical equilibrium with carbonic acid, which in turn is in equilibrium with bicarbonate. This is due to its polar bonds, and its reaction with water. The concentration of dissolved carbon dioxide therefore.
5 Important Sources of Greenhouse Gases
Web when carbon dioxide dissolves in water only some molecules of it will react with water. Measuring 27 ml of liquid (daudgtear ldnreiyc)________________3. Web the solubility of carbon dioxide in water decreases as the temperature is raised, and it is driven off into the atmosphere. Reaction of carbon dioxide with water is neglected. (carbonic acid), which is a weak acid.
Water could modulate the activity and selectivity of carbon dioxide
Web carbon dioxide is soluble in water, in which it reversibly forms. Hydrogen ions dissociate from the carbonic acid, to give bicarbonate (. Volume of solution does not change on dissolution of. Dissolved co, satisfies the equilibrium. This is due to its polar bonds, and its reaction with water.
Solved Carbon dioxide dissolves in water to form carbonic
Hydrogen ions dissociate from the carbonic acid, to give bicarbonate (. Web carbon dioxide dissolves in water to form carbonic acid. Most of it gets dissolved, co 2 remains loosely hydrated. Reaction of carbon dioxide with water is neglected. Dissolving anything is a physical change.
The reaction between carbon dioxide and water Experiment RSC Education
Carbon dioxide dissolves in water to form carbonic acid. Holding 100ml of water (ebkare)________________2. Web the solubility of carbon dioxide in water decreases as the temperature is raised, and it is driven off into the atmosphere. Volume of solution does not change on dissolution of. Web answer (1 of 2):
Carbon Cycle Jeopardy Template
(carbonic acid), which is a weak acid since its ionization in water is incomplete. Web co2 is quite soluble in water: 30% of all carbon dioxide is dissolved in water. Dissolved carbon dioxide is in chemical equilibrium with carbonic acid, which in turn is in equilibrium with bicarbonate. Estimate the thermodynamic equilibrium constant for this reaction using the ag, values.
Web Co2 Is Quite Soluble In Water:
Volume of solution does not change on dissolution of. Dissolved co, satisfies the equilibrium. Web cold water can dissolve more carbon dioxide than warm water. Measuring 27 ml of liquid (daudgtear ldnreiyc)________________3.
Web Carbon Dioxide Dissolves In Water To Form Carbonic Acid, Which Is Primarily Dissolved Co2.
And the rate at which carbon dioxide is removed from the air depends partly on the activities of water plants. Carbon dioxide dissolves in water to form carbonic acid. It reacts with water ( h 2 o) and forms carbonic acid ( h 2. Reaction of carbon dioxide with water is neglected.
Web Correct Option Is A) When Co 2 Dissolves In H 2O, Only Some Molecules React With H 2) To Form H 2Co 3.
Dissolved carbon dioxide is in chemical equilibrium with carbonic acid, which in turn is in equilibrium with bicarbonate. Dissolved co2 satisfies the equilibrium equation. Web carbon dioxide dissolves in water to form carbonic acid. Web carbon dioxide is soluble in water, in which it reversibly forms.
Web Expert Answer Transcribed Image Text:
Web when carbon dioxide dissolves in water only some molecules of it will react with water. The concentration of dissolved carbon dioxide therefore. This is due to its polar bonds, and its reaction with water. Most of it gets dissolved, co 2 remains loosely hydrated.