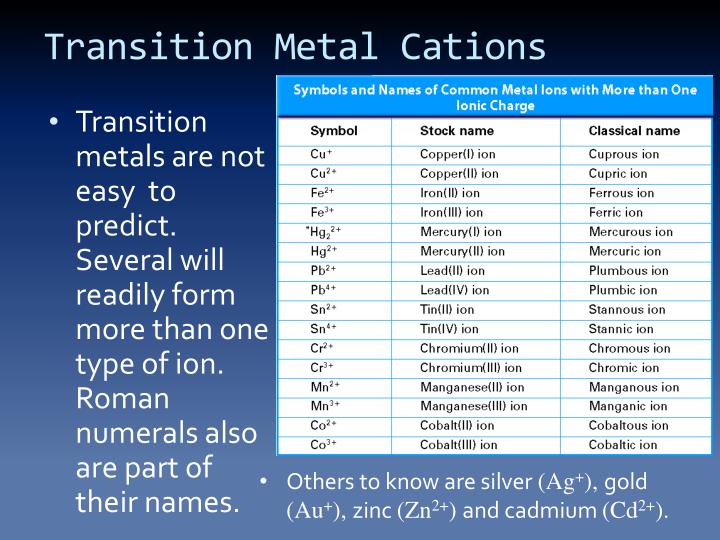
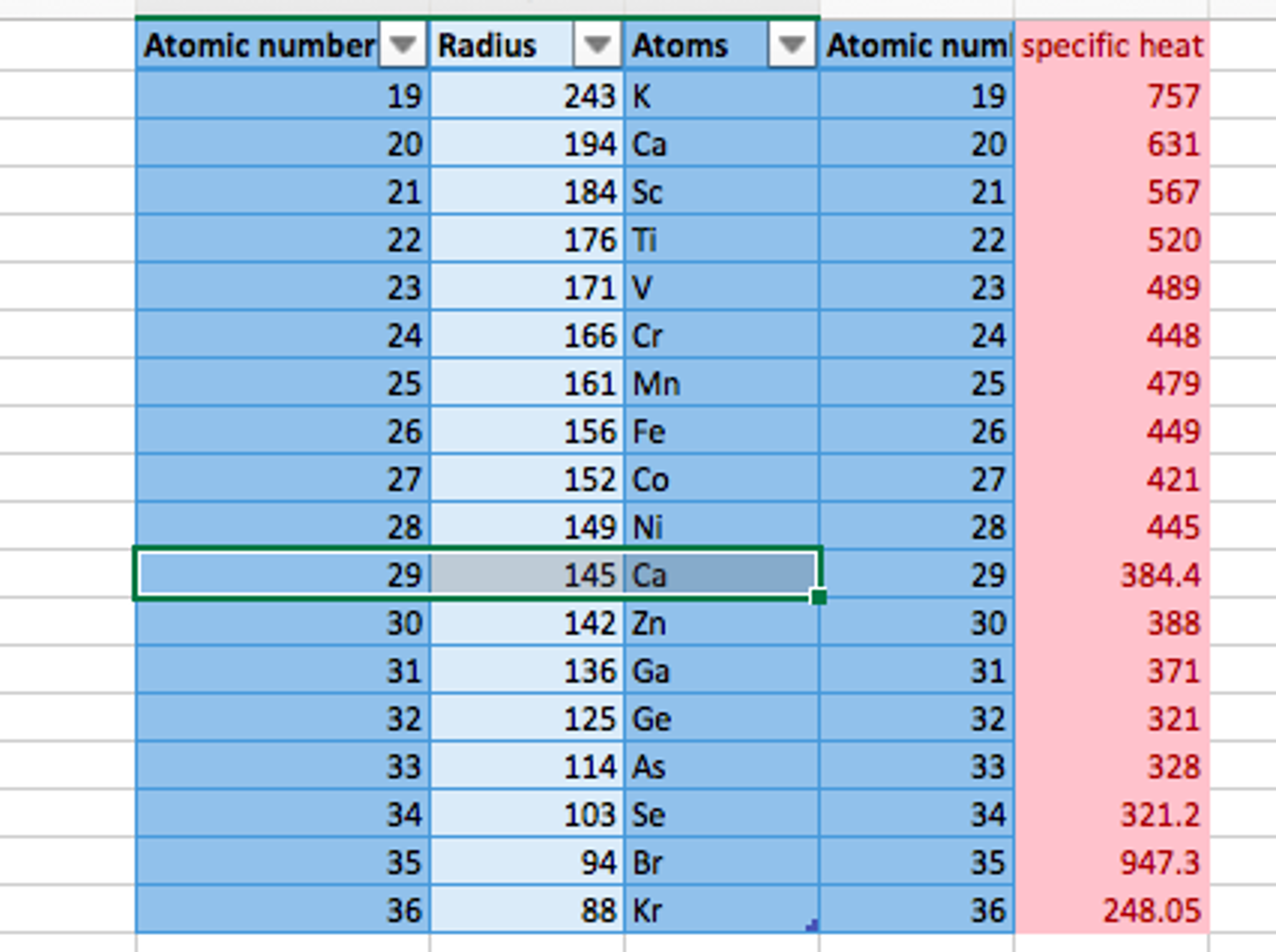
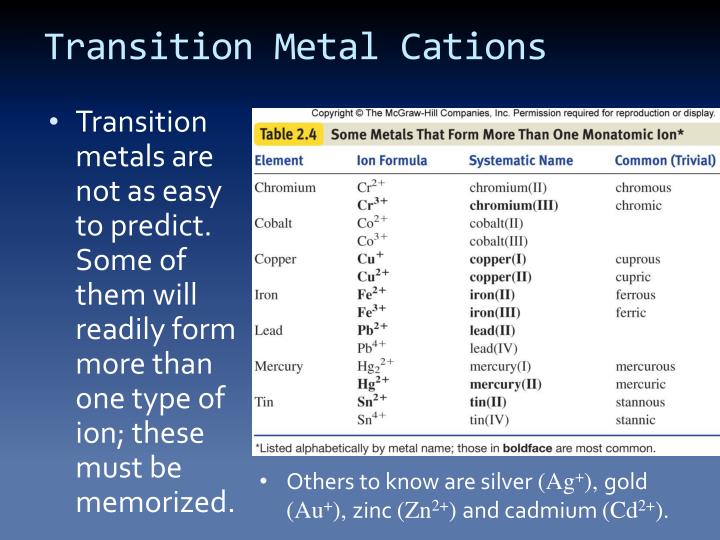
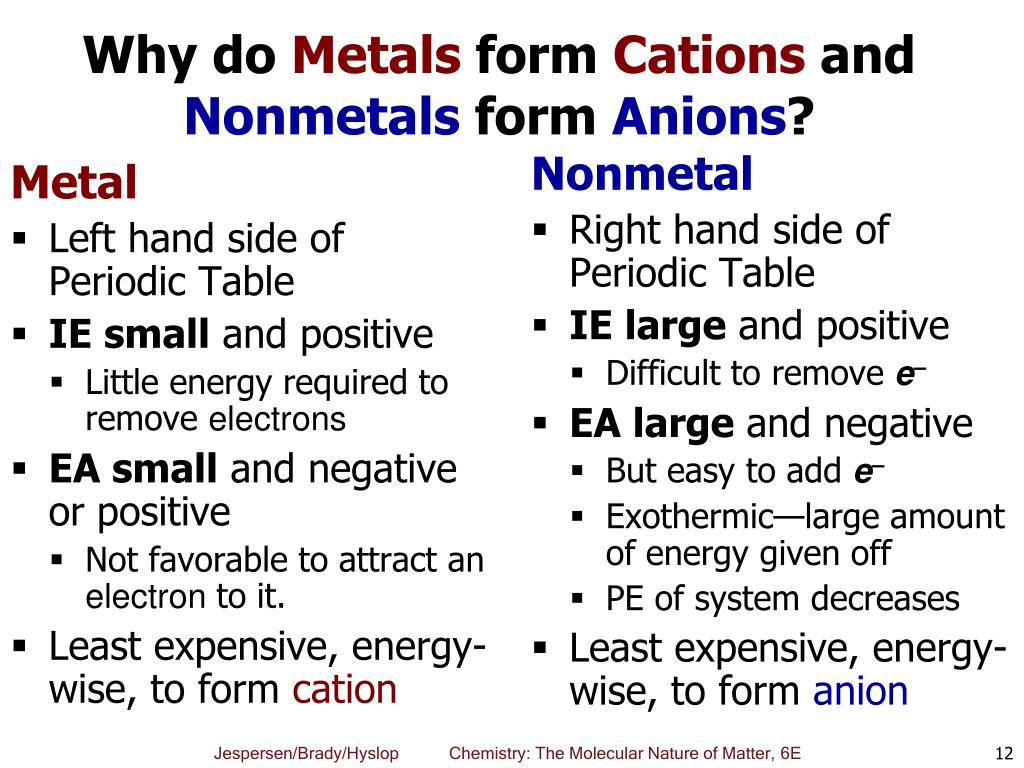
Do Metals Form Cations
Do Metals Form Cations - Web what do metals consist of? All chemical reactions are driven to gain more. What types of ions do the metallic and the nonmetallic elements form? Oxygen, nitrogen, sulfur), while most metals form cations (e.g. It has fewer electrons than protons. Web alkali metals and alkaline earth metals always form cations. Most other nonmetals typically form anions (e.g. And because of this behavior,. Closely packed cations and loosely held valence electrons rather than neutral atoms what can the valence electrons in a pure metal be modeled as? They lose one or more than one electron.
And because of this behavior,. Some examples of cations are calcium (ca 2+ ), potassium (k + ), hydrogen (h + ). Web having lost electrons, which are negatively charged, atoms of metals therefore gain a net positive charge i.e they become cations. Oxygen, nitrogen, sulfur), while most metals form cations (e.g. Transition metals often form ions wihout complete octets that's why all the stable ions are all cations. Pearson higher level chemistry textbook, 2nd edition. Web cations are positively charged ions. They lose one or more than one electron. Writing chemical formulas when writing the formula of a compound, the cation is listed before the anion. Cl atoms gain 1 electron, therefore making them anions.
Most other nonmetals typically form anions (e.g. Cl atoms gain 1 electron, therefore making them anions. Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+. Web what do metals consist of? And because of this behavior,. Web having lost electrons, which are negatively charged, atoms of metals therefore gain a net positive charge i.e they become cations. Writing chemical formulas when writing the formula of a compound, the cation is listed before the anion. Web halogens always form anions, alkali metals and alkaline earth metals always form cations. You can also tell that they form cations because some. They are formed when a metal loses its electrons.
Solved Question 6 (1 point) Why do metals tend to form
Web cations are atoms that contain a positive charge, and they are formed when the atoms lose electrons which are negatively charged. They lose one or more than one electron. It has fewer electrons than protons. What types of ions do the metallic and the nonmetallic elements form? They are formed when a metal loses its electrons.
PPT Naming IONS & formulas for Ionic Compounds PowerPoint
Web halogens always form anions, alkali metals and alkaline earth metals always form cations. Web having lost electrons, which are negatively charged, atoms of metals therefore gain a net positive charge i.e they become cations. Web cations are atoms that contain a positive charge, and they are formed when the atoms lose electrons which are negatively charged. Web a metal.
Chem matters ch6_ionic_bond
Web halogens always form anions, alkali metals and alkaline earth metals always form cations. Web what do metals consist of? Most other nonmetals typically form anions (e.g. And because of this behavior,. They lose one or more than one electron.
PPT 1 Name the ions formed by these elements and classify them as
Therefore, they possess a net positive charge. Web halogens always form anions, alkali metals and alkaline earth metals always form cations. Web cations are atoms that contain a positive charge, and they are formed when the atoms lose electrons which are negatively charged. Cl atoms gain 1 electron, therefore making them anions. This is the typical behavior for many metal.
is incorreer? all s area eleseis erand e are metals b, all p area
You can also tell that they form cations because some. Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+. This is the typical behavior for many metal substances. What types of ions do the metallic and the nonmetallic elements form? The solvation number , n.
Form a table of alkaline earth metal cations and the
Web halogens always form anions, alkali metals and alkaline earth metals always form cations. The solvation number , n , determined by a variety of experimental methods is 4 for li + and be 2+ and 6 for most elements in periods 3. Writing chemical formulas when writing the formula of a compound, the cation is listed before the anion..
PPT IONS PowerPoint Presentation ID2435906
Most other nonmetals typically form anions (e.g. What types of ions do the metallic and the nonmetallic elements form? Oxygen, nitrogen, sulfur), while most metals form cations (e.g. Some examples of cations are calcium (ca 2+ ), potassium (k + ), hydrogen (h + ). Web having lost electrons, which are negatively charged, atoms of metals therefore gain a net.
PPT Chapter 9 The Basics of Chemical Bonding PowerPoint Presentation
Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+. Web what do metals consist of? Writing chemical formulas when writing the formula of a compound, the cation is listed before the anion. The solvation number , n , determined by a variety of experimental methods.
Do metals form anions or cations quizlet? Book Vea
And because of this behavior,. Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+. By catrin brown and mike ford. Most other nonmetals typically form anions (e.g. Oxygen, nitrogen, sulfur), while most metals form cations (e.g.
Oxygen, Nitrogen, Sulfur), While Most Metals Form Cations (E.g.
They lose one or more than one electron. Web alkali metals and alkaline earth metals always form cations. It has fewer electrons than protons. And because of this behavior,.
All Chemical Reactions Are Driven To Gain More.
Web cations are atoms that contain a positive charge, and they are formed when the atoms lose electrons which are negatively charged. Web metals tend to form cations while nonmetals tend to form anions. Web what do metals consist of? Closely packed cations and loosely held valence electrons rather than neutral atoms what can the valence electrons in a pure metal be modeled as?
Pearson Higher Level Chemistry Textbook, 2Nd Edition.
Some examples of cations are calcium (ca 2+ ), potassium (k + ), hydrogen (h + ). This is the typical behavior for many metal substances. Web having lost electrons, which are negatively charged, atoms of metals therefore gain a net positive charge i.e they become cations. They are formed when a metal loses its electrons.
Most Other Nonmetals Typically Form Anions (E.g.
Transition metals often form ions wihout complete octets that's why all the stable ions are all cations. The solvation number , n , determined by a variety of experimental methods is 4 for li + and be 2+ and 6 for most elements in periods 3. Web halogens always form anions, alkali metals and alkaline earth metals always form cations. You can also tell that they form cations because some.