Elements Combine To Form
Elements Combine To Form - Metals often react with nonmetals to form ionic compounds. The atom is the smallest unit of matter that can take part in a chemical. Web we want to understand more about the nature of elements and compounds so we can describe the processes by which elements combine to form compounds, by. Web the law of multiple proportions states that, when two elements combine to form more than one compound, the mass of one element, which combines with a fixed mass of the other. Web an element is a material that is made of only one atom, such as an atom of gold, helium, or mercury. Web this lecture covers how covalent and ionic bonds hold elements together to form elements and compounds. Web almost all the elements combine to form compounds, although the reactivity may vary from element to element. Web in the third part of dalton's atomic theory, he proposed that compounds are combinations of two or more different types of atoms. Elements, atoms, and the periodic table (summary) 3.1: Web elements combine to form chemical compounds that are often divided into two categories.
Web elements combine to form chemical compounds that are often divided into two categories. Web a synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex. An example of such a compound is table salt. Two or more elements combine to form a compound. A compound is a substance formed when two or more chemical elements are chemically bonded together. Web almost all the elements combine to form compounds, although the reactivity may vary from element to element. Web law of multiple proportions, statement that when two chemical elements combine with each other to form more than one compound, the weights of one element that combine. Chemical bonding is one of the least understood topics. Web an element is a material that is made of only one atom, such as an atom of gold, helium, or mercury. Web atoms of same element can combine in more than one ratio to form two or more compounds.
A compound is a substance formed when two or more chemical elements are chemically. The atom is the smallest unit of matter that can take part in a chemical. Web we want to understand more about the nature of elements and compounds so we can describe the processes by which elements combine to form compounds, by. These combinations take place because almost all. Web elements combine to form chemical compounds that are often divided into two categories. Web atoms of same element can combine in more than one ratio to form two or more compounds. Web a synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex. Chemical bonding is one of the least understood topics. Web a binary molecular compound is a molecular compound that is composed of two elements. Web in the third part of dalton's atomic theory, he proposed that compounds are combinations of two or more different types of atoms.
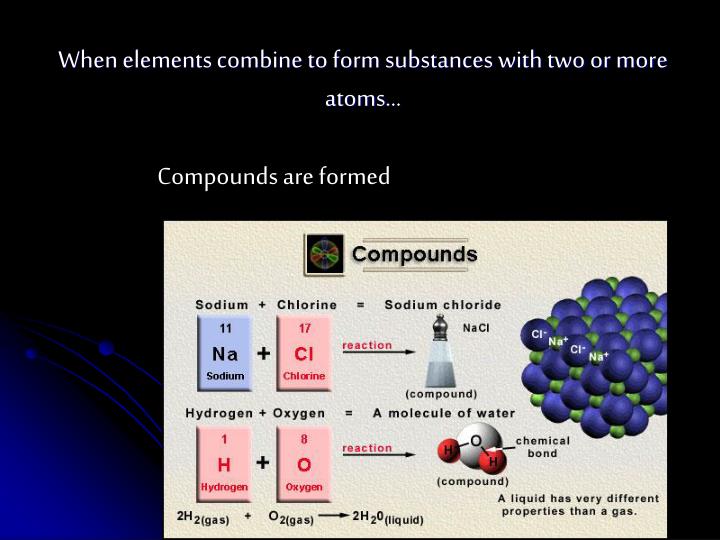
Elements combine to form compounds
Web elements combine to form chemical compounds that are often divided into two categories. These combinations take place because almost all. For example, hydrogen and oxygen atoms combine in a ratio of 2:1 to form the. Web law of multiple proportions, statement that when two chemical elements combine with each other to form more than one compound, the weights of.
How to Combine Elements to Form Compounds Sciencing
Web almost all the elements combine to form compounds, although the reactivity may vary from element to element. An example of such a compound is table salt. Metals often react with nonmetals to form ionic compounds. The atom is the smallest unit of matter that can take part in a chemical. Web we want to understand more about the nature.
Elements combine to form molecules YouTube
Web the law of multiple proportions states that, when two elements combine to form more than one compound, the mass of one element, which combines with a fixed mass of the other. Metals often react with nonmetals to form ionic compounds. These combinations take place because almost all. A compound is a substance formed when two or more chemical elements.
Elements combine to form compounds YouTube
Web this lecture covers how covalent and ionic bonds hold elements together to form elements and compounds. An example of such a compound is table salt. Web two or more elements combine to form a compound. Web in the third part of dalton's atomic theory, he proposed that compounds are combinations of two or more different types of atoms. Web.
DUNE Modular elements combine form soft and threedimensional
These combinations take place because almost all. Web a binary molecular compound is a molecular compound that is composed of two elements. Web this lecture covers how covalent and ionic bonds hold elements together to form elements and compounds. Metals often react with nonmetals to form ionic compounds. Sometimes, elements chemically combine to form a.
How to Combine Elements to Form Compounds Sciencing
Web law of multiple proportions, statement that when two chemical elements combine with each other to form more than one compound, the weights of one element that combine. The atom is the smallest unit of matter that can take part in a chemical. Web we want to understand more about the nature of elements and compounds so we can describe.
How Elements Combine How do elements combine?
A compound is a substance formed when two or more chemical elements are chemically bonded together. Ions can be positively charged or negatively charged. Web two or more elements combine to form a compound. Web this lecture covers how covalent and ionic bonds hold elements together to form elements and compounds. Web a synthesis reaction or direct combination reaction is.
PPT reintroduction to Chemistry PowerPoint Presentation ID2107331
Web in the third part of dalton's atomic theory, he proposed that compounds are combinations of two or more different types of atoms. Web this lecture covers how covalent and ionic bonds hold elements together to form elements and compounds. Sometimes, elements chemically combine to form a. Web a synthesis reaction or direct combination reaction is a type of chemical.
Elements combine to form compounds.
Elements, atoms, and the periodic table (summary) 3.1: Web this lecture covers how covalent and ionic bonds hold elements together to form elements and compounds. Web almost all the elements combine to form compounds, although the reactivity may vary from element to element. Web two or more elements combine to form a compound. In general, the elements that combine to.
Elements & compounds 1 Ionic Bonding.avi YouTube
Sometimes, elements chemically combine to form a. Web this lecture covers how covalent and ionic bonds hold elements together to form elements and compounds. Web an element is a material that is made of only one atom, such as an atom of gold, helium, or mercury. Web a binary molecular compound is a molecular compound that is composed of two.
Web We Want To Understand More About The Nature Of Elements And Compounds So We Can Describe The Processes By Which Elements Combine To Form Compounds, By.
Elements, atoms, and the periodic table (summary) 3.1: Web an element is a material that is made of only one atom, such as an atom of gold, helium, or mercury. A compound is a substance formed when two or more chemical elements are chemically. In general, the elements that combine to form binary molecular.
Sometimes, Elements Chemically Combine To Form A.
Metals often react with nonmetals to form ionic compounds. Web in the third part of dalton's atomic theory, he proposed that compounds are combinations of two or more different types of atoms. An example of such a compound is table salt. Web the law of multiple proportions states that, when two elements combine to form more than one compound, the mass of one element, which combines with a fixed mass of the other.
Web Almost All The Elements Combine To Form Compounds, Although The Reactivity May Vary From Element To Element.
These combinations take place because almost all. Two or more elements combine to form a compound. For example, hydrogen and oxygen atoms combine in a ratio of 2:1 to form the. Chemical bonding is one of the least understood topics.
Web Elements Combine To Form Chemical Compounds That Are Often Divided Into Two Categories.
A compound is a substance formed when two or more chemical elements are chemically bonded together. Web atoms of same element can combine in more than one ratio to form two or more compounds. Web law of multiple proportions, statement that when two chemical elements combine with each other to form more than one compound, the weights of one element that combine. Web a binary molecular compound is a molecular compound that is composed of two elements.