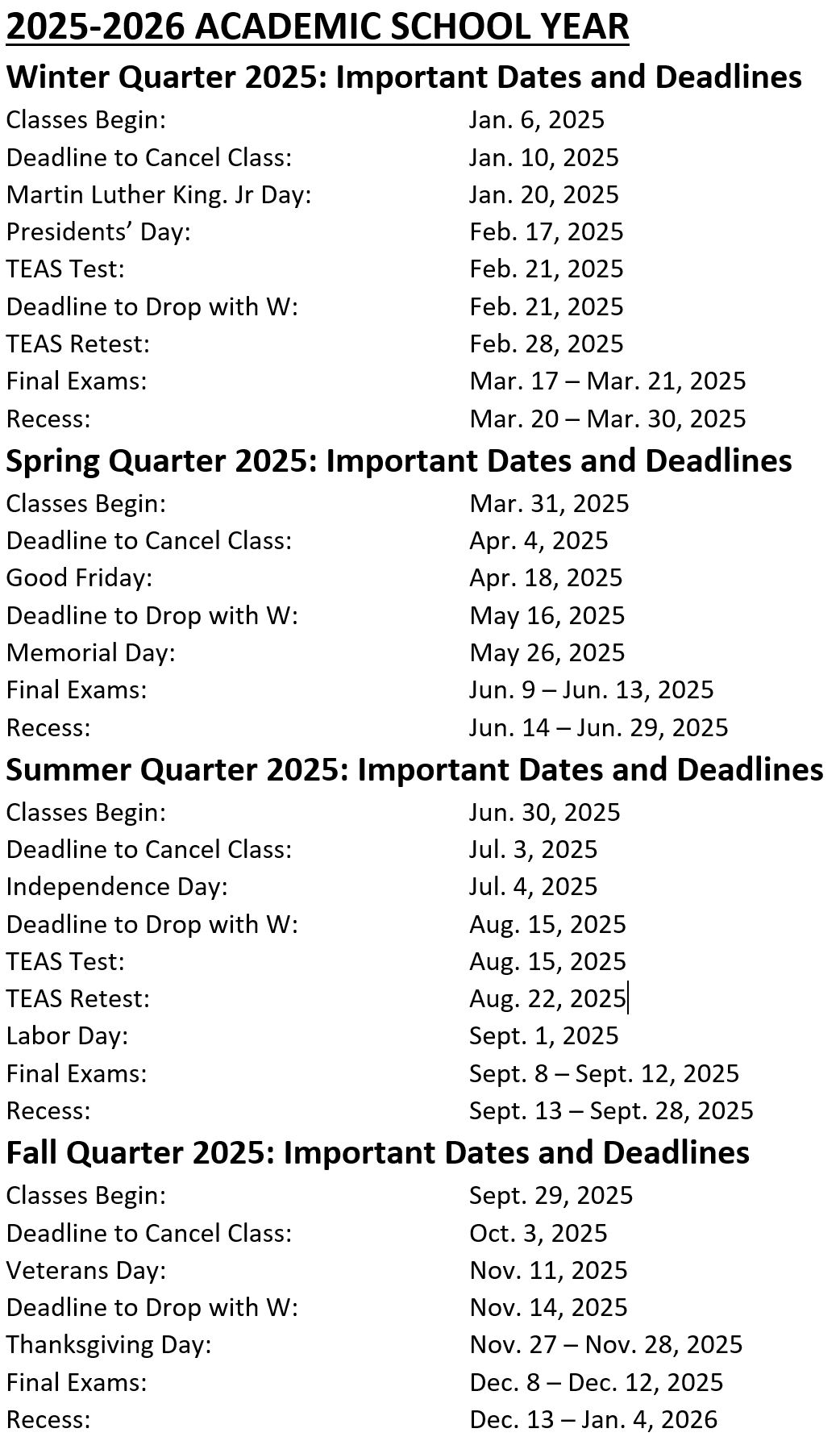
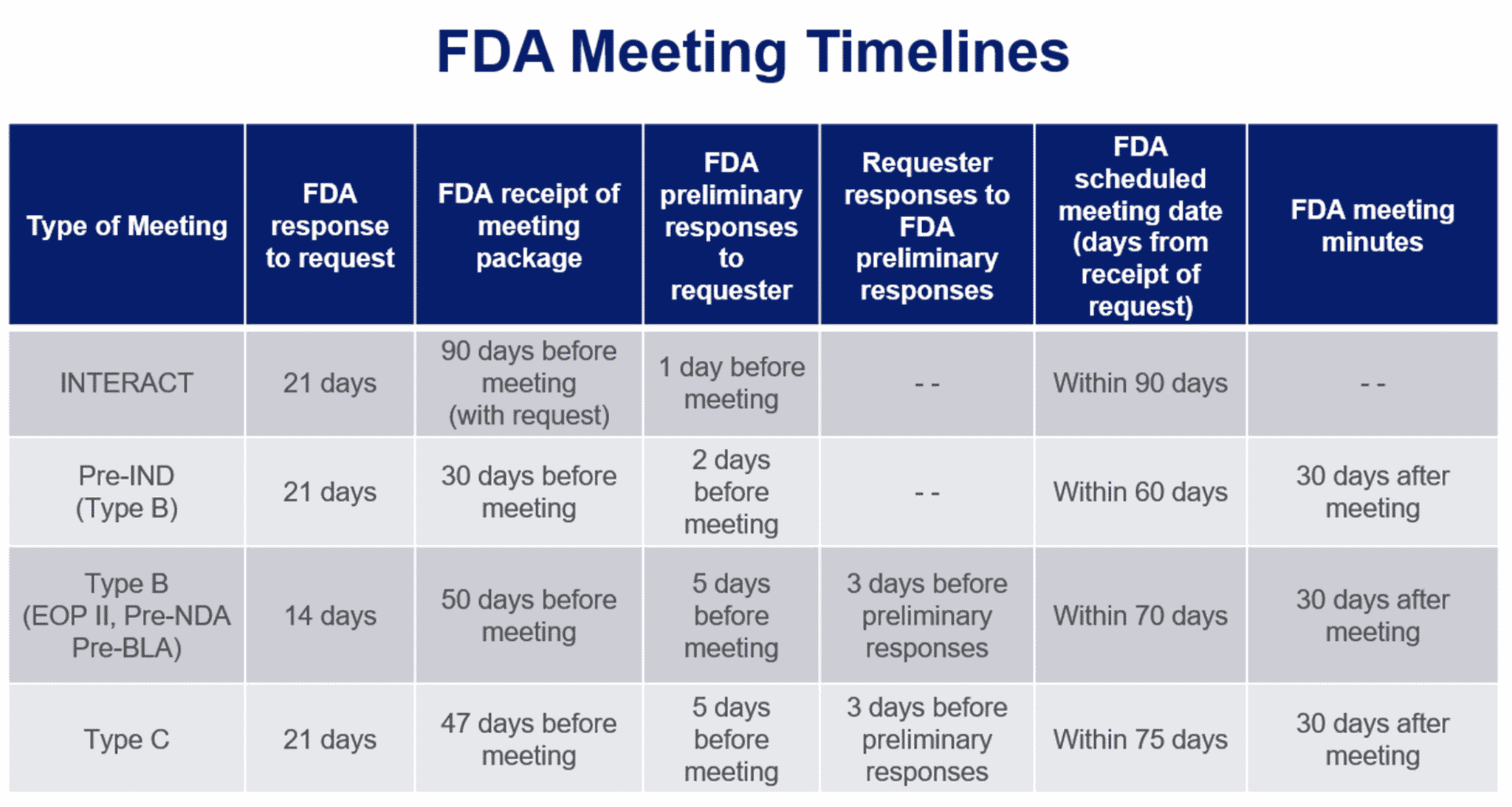
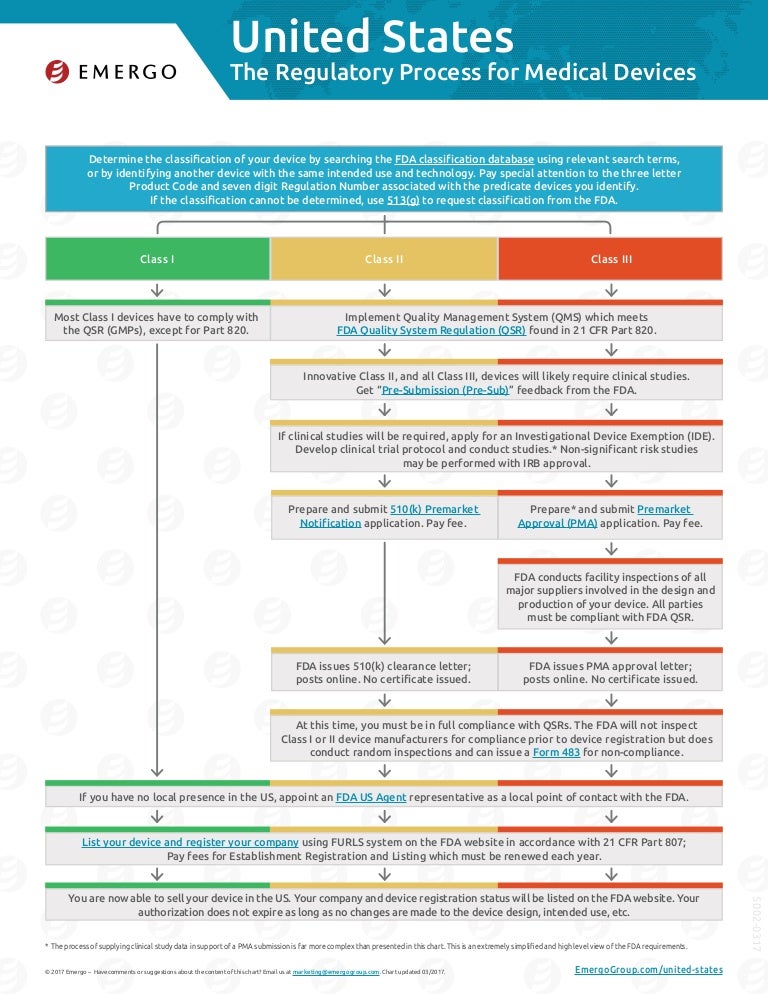
Fda Approval Calendar
Fda Approval Calendar - See the drug name, active. See the list of pending and approved drugs, their indications, rivals and market potential. Web the pdufa date refers to the date the food and drug administration (fda) are expected to deliver their decision whether or not to approve a company’s new drug application. Last year the fda’s center for drug evaluation. Web our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. There were one or two controversial decisions and a slight. (embc) has received 510 (k) clearance from the fda for its disposable insulin delivery system. See the latest approval reports, postmarket safety. Skip to in this section menu. Web 2022 fda approvals.
Priority review applications and advisory committee meeting dates included. The fda approved 37 novel drugs in 2022, the fewest to pass regulatory scrutiny since 2016. Follow fda on linkedin view fda videos on. Last year the fda’s center for drug evaluation. There were one or two controversial decisions and a slight. Low flat feemultilingual supportquick & easy compliance24/7 live help Web see the list of new molecular entities and new therapeutic biological products approved by fda in 2022, organized by calendar year. Web the pdufa date refers to the date the food and drug administration (fda) are expected to deliver their decision whether or not to approve a company’s new drug application. Web pdufa dates (fda approval) for all us publicly listed biotech companies. Indicated for adults who require insulin to manage.
These include clinical trials, commercial, and fda or other regulatory events. Priority review applications and advisory committee meeting dates included. Web our free fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including clinical trials, regulatory events, and. Web boards, policy & regulation category · september 19, 2024 us fda declines to approve vanda's stomach condition drug. Skip to in this section menu. Web the catalyst calendar is a chronological calendar of biotech events that could move the stock price. Web 2022 fda approvals. Web find approval information by product type, such as drugs, biologics, medical devices, animal drugs, and food additives. Fda approves vorasidenib for grade 2 astrocytoma or oligodendroglioma with a susceptible idh1 or idh2 mutation. The table is updated regularly and.
Fda Approval Calendar 2023 Everything You Need To Know August 2023
Web see the list of new molecular entities and new therapeutic biological products approved by fda in 2022, organized by calendar year. Web find approval information by product type, such as drugs, biologics, medical devices, animal drugs, and food additives. Skip to in this section menu. (embc) has received 510 (k) clearance from the fda for its disposable insulin delivery.
Fda Drug Approval Calendar Printable Word Searches
Find out the drug name, active ingredient,. Web see the list of new molecular entities and new therapeutic biological products approved by fda in 2022, organized by calendar year. (embc) has received 510 (k) clearance from the fda for its disposable insulin delivery system. The fda approved 37 novel drugs in 2022, the fewest to pass regulatory scrutiny since 2016..
Fda Approval Calendar Harri Pepita
Web download a pdf document with a list of new molecular entities and new therapeutic biological products approved by fda in 2023. See the latest approval reports, postmarket safety. On august 6, 2024, the. Web see the list of new molecular entities and new therapeutic biological products approved by fda in 2022, organized by calendar year. Web find out the.
Fda Approval Calendar Harri Pepita
Find out the drug name, active ingredient,. Web pdufa dates (fda approval) for all us publicly listed biotech companies. Web see the list of new molecular entities and new therapeutic biological products approved by fda in 2022, organized by calendar year. Web our free fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech.
Fda Approval Calendar 2025 barbi benoite
See the list of pending and approved drugs, their indications, rivals and market potential. Low flat feemultilingual supportquick & easy compliance24/7 live help Fda approves vorasidenib for grade 2 astrocytoma or oligodendroglioma with a susceptible idh1 or idh2 mutation. Web find out the latest fda approval, pdufa and panel review dates for biotech stocks. Skip to in this section menu.
FDA Calendar FDA Tracker
Low flat feemultilingual supportquick & easy compliance24/7 live help Priority review applications and advisory committee meeting dates included. Fda approves vorasidenib for grade 2 astrocytoma or oligodendroglioma with a susceptible idh1 or idh2 mutation. Web find approval information by product type, such as drugs, biologics, medical devices, animal drugs, and food additives. Fda previously granted emergency use authorization on december.
Fda Meeting Calendar Alyse Bertine
Fda approves vorasidenib for grade 2 astrocytoma or oligodendroglioma with a susceptible idh1 or idh2 mutation. Web find approval information by product type, such as drugs, biologics, medical devices, animal drugs, and food additives. Web find out the latest fda approval, pdufa and panel review dates for biotech stocks. The table is updated regularly and. Web our free fda calendar.
US FDA medical device approval chart Emergo
Web find approval information by product type, such as drugs, biologics, medical devices, animal drugs, and food additives. There were one or two controversial decisions and a slight. Web boards, policy & regulation category · september 19, 2024 us fda declines to approve vanda's stomach condition drug. The table is updated regularly and. Web find out the latest fda approval,.
FDA Calendar FDA Tracker
Web find out the latest fda approval, pdufa and panel review dates for biotech stocks. Web see the list of new molecular entities and new therapeutic biological products approved by fda in 2022, organized by calendar year. Web our free fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including.
Fda Approval Calendar Harri Pepita
Web nda and bla calendar year approvals. Web find approval information by product type, such as drugs, biologics, medical devices, animal drugs, and food additives. These include clinical trials, commercial, and fda or other regulatory events. Web find out the list of new drugs approved by the fda in 2024, with their names, active ingredients, approval dates, and indications. Indicated.
There Were One Or Two Controversial Decisions And A Slight.
Web see the list of new molecular entities and new therapeutic biological products approved by fda in 2022, organized by calendar year. Skip to in this section menu. These include clinical trials, commercial, and fda or other regulatory events. Web find approval information by product type, such as drugs, biologics, medical devices, animal drugs, and food additives.
Web Benzinga's Fda Calendar Shows Historical Fda Data, Upcoming Dates That Companies Will Be Impacted By The Fda And Ranges Of Dates.
Web find out the latest fda approval, pdufa and panel review dates for biotech stocks. Indicated for adults who require insulin to manage. Web nda and bla calendar year approvals. Follow fda on linkedin view fda videos on.
On August 6, 2024, The.
Web download a pdf document with a list of new molecular entities and new therapeutic biological products approved by fda in 2023. Web our free fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including clinical trials, regulatory events, and. The fda approved 37 novel drugs in 2022, the fewest to pass regulatory scrutiny since 2016. Find out the drug name, active ingredient,.
See The List Of Pending And Approved Drugs, Their Indications, Rivals And Market Potential.
Fda previously granted emergency use authorization on december 11, 2020, for individuals. Low flat feemultilingual supportquick & easy compliance24/7 live help Web pdufa dates (fda approval) for all us publicly listed biotech companies. Fda approves vorasidenib for grade 2 astrocytoma or oligodendroglioma with a susceptible idh1 or idh2 mutation.