Fda Form 2579
Fda Form 2579 - Read the following instructions to use cocodoc to start editing and filling out your fda 2579: Electronic product radiation control (eprc) reports and records may be submitted by any of the following methods:. Follow these simple guidelines to get fda 2579 form printable completely ready for sending: Use the following instructions to download the form if. Are of the type called for by the. Web how to edit and sign fda 2579 online. F pdf form is not working you may. 4 systems records and reports. Assemblers will still be required to submit a copy to the. Completing the report of assembly form fda 2579.mar 24, 2015.
Web follow these fast steps to edit the pdf fda 2579 online free of charge: Register and log in to your account. Save or instantly send your ready documents. Firstly, look for the “get form” button and click. Web requesting forms from the warehouse. Web enter your official identification and contact details. The report of assembly (form 2579) represents the assemblers certification that the system or component(s): 4 systems records and reports. Web how to edit and sign fda 2579 online. Web how to submit electronic product reports:
The report of assembly (form 2579) represents the assemblers certification that the system or component(s): F pdf form is not working you may. Web how to edit and sign fda 2579 online. Double check all the fillable fields to ensure complete. Easily fill out pdf blank, edit, and sign them. Are of the type called for by the. Assemblers will still be required to submit a copy to the. 4 systems records and reports. Web up to $3 cash back reader can be downloaded for free at.may 17, 2011. Web complete fda 2579 online with us legal forms.
Form FDA 2481 Medicated Feeds Inspection Report Free Download
Electronic product radiation control (eprc) reports and records may be submitted by any of the following methods:. The report of assembly (form 2579) represents the assemblers certification that the system or component(s): Assemblers will still be required to submit a copy to the. Get the document you want in our library of legal forms. Web up to $3 cash back.
Form FDA 37191 Report of Inspection for Compliance Free Download
Easily fill out pdf blank, edit, and sign them. Web it only takes a few minutes. Web how to submit electronic product reports: Save or instantly send your ready documents. Web follow these fast steps to edit the pdf fda 2579 online free of charge:
Preparation of the Food and Drug Administration Form 2579
Save or instantly send your ready documents. Web it only takes a few minutes. Web up to $3 cash back reader can be downloaded for free at.may 17, 2011. Sign in to the editor using your credentials or click create free account. Web enter your official identification and contact details.
free 11 sample dental consent forms in pdf word x ray consent form
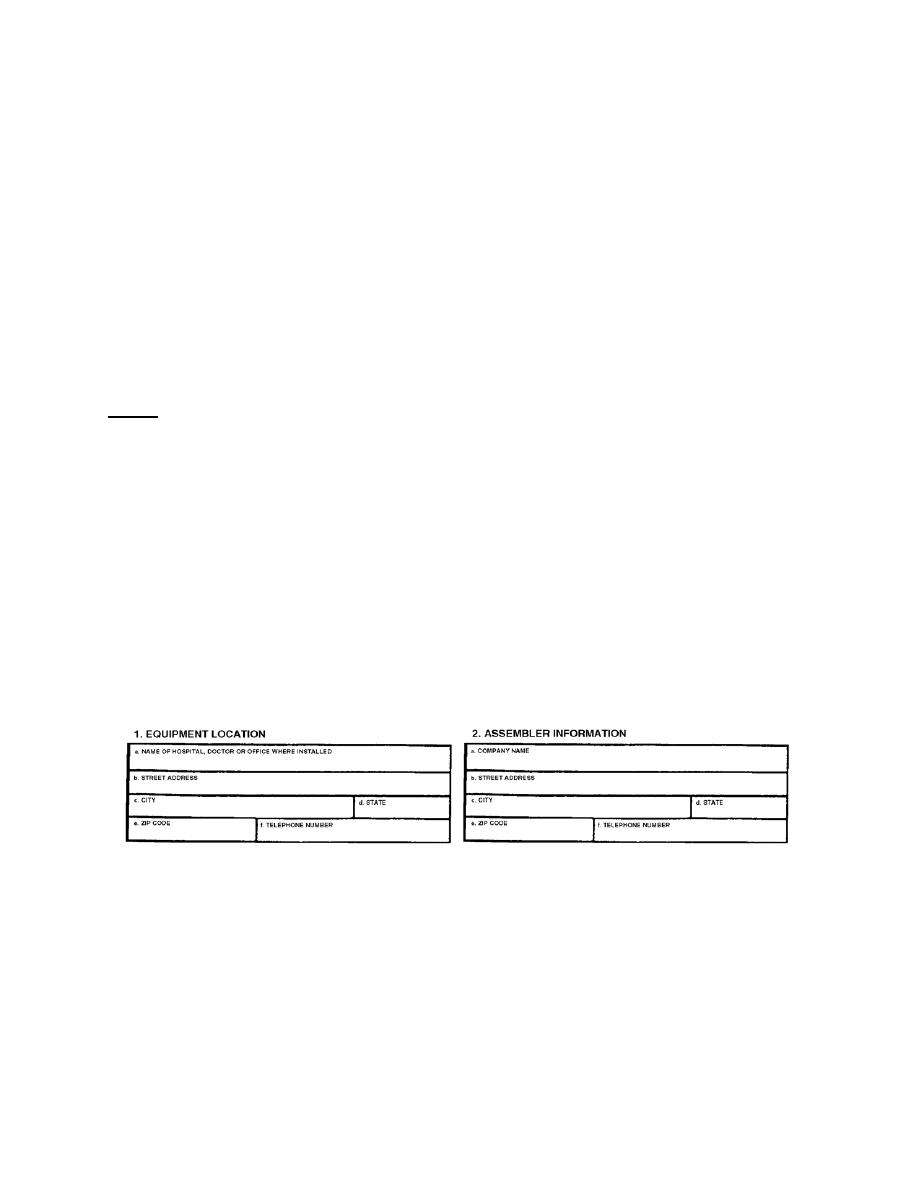
Web the report of assembly (form fda 2579) represents the assemblers’ certification that the system or component (s) are of the type called for by the standard (i.e., certified), have. Web how to submit electronic product reports: Web how to edit and sign fda 2579 online. Completing the report of assembly form fda 2579.mar 24, 2015. Web it only takes.
Form FDA 3356 Establishment Registration and Listing for HCT/Ps Free
Electronic product radiation control (eprc) reports and records may be submitted by any of the following methods:. Web it only takes a few minutes. Firstly, look for the “get form” button and click. Web in this rulemaking, fda is removing the requirement to submit a copy of form fda 2579 to fda. Register and log in to your account.
Form FDA 37191 Report of Inspection for Compliance Free Download
Web how to edit and sign fda 2579 online. Completing the report of assembly form fda 2579.mar 24, 2015. Double check all the fillable fields to ensure complete. Assemblers will still be required to submit a copy to the. Web what is an fda form 2579?
Fda 2579 Form ≡ Fill Out Printable PDF Forms Online
F pdf form is not working you may. Food and drug administration and to the virginia department of health. Save or instantly send your ready documents. Follow these simple guidelines to get fda 2579 form printable completely ready for sending: Register and log in to your account.
Form 10F Filled Sample Fill Out and Sign Printable PDF Template signNow
4 systems records and reports. Web requesting forms from the warehouse. Save or instantly send your ready documents. Web how to edit and sign fda 2579 online. Sign in to the editor using your credentials or click create free account.
Fda 2579 Form Fill Out and Sign Printable PDF Template signNow
Use the following instructions to download the form if. Save or instantly send your ready documents. Sign in to the editor using your credentials or click create free account. Food and drug administration and to the virginia department of health. Read the following instructions to use cocodoc to start editing and filling out your fda 2579:
Form FDA 2359d Report of Certification Free Download
Assemblers will still be required to submit a copy to the. Use the following instructions to download the form if. Web how to submit electronic product reports: Double check all the fillable fields to ensure complete. F pdf form is not working you may.
Web Complete Fda 2579 Online With Us Legal Forms.
Save or instantly send your ready documents. Apply a check mark to indicate the answer wherever needed. Are of the type called for by the. Use the following instructions to download the form if.
Completing The Report Of Assembly Form Fda 2579.Mar 24, 2015.
Read the following instructions to use cocodoc to start editing and filling out your fda 2579: Web enter your official identification and contact details. Web the report of assembly (form fda 2579) represents the assemblers’ certification that the system or component (s) are of the type called for by the standard (i.e., certified), have. Double check all the fillable fields to ensure complete.
4 Systems Records And Reports.
Web it only takes a few minutes. F pdf form is not working you may. Food and drug administration and to the virginia department of health. Get the document you want in our library of legal forms.
Web In This Rulemaking, Fda Is Removing The Requirement To Submit A Copy Of Form Fda 2579 To Fda.
Web follow these fast steps to edit the pdf fda 2579 online free of charge: Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Electronic product radiation control (eprc) reports and records may be submitted by any of the following methods:. Follow these simple guidelines to get fda 2579 form printable completely ready for sending: