How Many Bonds Can Boron Form
How Many Bonds Can Boron Form - Boron, b, has an electronic configuration of 1s2 2s2 2p1. The most familiar borate is sodium. An example is bh3, boron trihydride. Web see answer (1) best answer. Many of the boranes readily oxidise on. Bf3, boron triflouride, is another. Web typical, boron forms 3 covalent bonds. Web boron has a charge of 5. Adhesives, cement, disinfectants, fertilizers, etc. Web how many bonds can boron form?
These compounds do not occur in nature. Boron, b, has an electronic configuration of 1s2 2s2 2p1. Typically, boron forms 3 covalent bonds. Web how many bonds can boron form? With 3 electrons boron can form 3 single covalent bonds. Web how many single bonds can boron form? This is balanced by 5 electrons. Web because there are only three valence electrons boron forms three bonds with other elements. We know that boron is an element whose atomic number is 5. Boron can form a fourth covalent bond and thus acquire a formal negative charge.
Many of the boranes readily oxidise on. Web typically, boron forms 3 covalent bonds. An example is bh3, boron trihydride. We know that boron is an element whose atomic number is 5. Web boranes are chemical compounds of boron and hydrogen, with the generic formula of b x h y. Boron can form a fourth covalent bond and thus acquire a formal negative charge. Boron trifluoride consists of a central boron atom with three single bonds to fluorine atoms(see figure below). The valence electrons may participate in. Web because there are only three valence electrons boron forms three bonds with other elements. This is balanced by 5 electrons.
Polar Bond
Web because there are only three valence electrons boron forms three bonds with other elements. These compounds do not occur in nature. Web instead, boron forms unique and intricate structures that contain multicenter bonds, in which a pair of electrons holds together three or more atoms. Boron trifluoride consists of a central boron atom with three single bonds to fluorine.
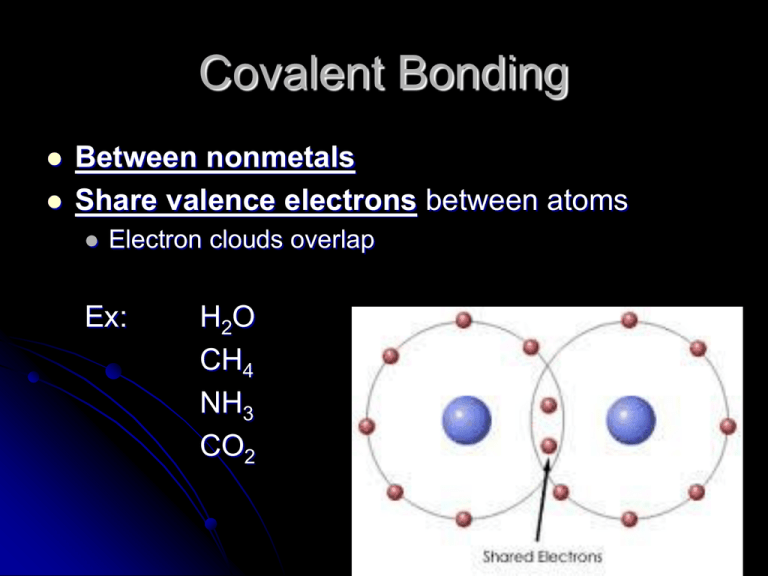
chemistry Why does boron form only covalent compounds
This is balanced by 5 electrons. Web for example, beryllium can form two covalent bonds, resulting in only four electrons in its valence shell: An example is bh3, boron trihydride. A derivative of a borane in which one or more hydrogen atoms have been replaced by. Two of them are core electrons and the remaining 3 are valence electrons.
Boron Facts Periodic Table of the Elements
Adhesives, cement, disinfectants, fertilizers, etc. The valence electrons may participate in. Web typically, boron forms 3 covalent bonds. The most familiar borate is sodium. Bf3, boron triflouride, is another.
Unprecedented formation of a boronboron covalent bond opens a new
Web how many single bonds can boron form? The valence electrons may participate in. Web typical, boron forms 3 covalent bonds. Typically, boron forms 3 covalent bonds. Bf3, boron triflouride, is another.
How Many Bonds Can Nitrogen Form Jacks Of Science
Web typical, boron forms 3 covalent bonds. Boron can form a fourth covalent bond and thus acquire an formal negative charge. Two of them are core electrons and the remaining 3 are valence electrons. We know that boron is an element whose atomic number is 5. Bf3, boron triflouride, is another.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
The electrons are arranged in shells, with 2 on the first shell and 3 on the. We know that boron is an element whose atomic number is 5. Boron, b, has an electronic configuration of 1s2 2s2 2p1. An example is bh3, boron trihydride. Boron trifluoride consists of a central boron atom with three single bonds to fluorine atoms(see figure.
Properties Of Metalloids For Chemistry Class Science Trends
These compounds do not occur in nature. With 3 electrons boron can form 3 single covalent bonds. An example is bh3, boron trihydride. We know that boron is an element whose atomic number is 5. Typically, boron forms 3 covalent bonds.
__TOP__ How Many Covalent Bonds Can Chlorine Form
Boron commonly makes only three covalent bonds,. With 3 electrons boron can form 3 single covalent bonds. Adhesives, cement, disinfectants, fertilizers, etc. The electrons are arranged in shells, with 2 on the first shell and 3 on the. Web boranes are chemical compounds of boron and hydrogen, with the generic formula of b x h y.
How Many Bonds Can Nitrogen Form Jacks Of Science
Two of them are core electrons and the remaining 3 are valence electrons. Boron trifluoride consists of a central boron atom with three single bonds to fluorine atoms(see figure below). Web for example, beryllium can form two covalent bonds, resulting in only four electrons in its valence shell: Adhesives, cement, disinfectants, fertilizers, etc. This is balanced by 5 electrons.
boron Properties, Uses, & Facts Britannica
Web for example, beryllium can form two covalent bonds, resulting in only four electrons in its valence shell: The most familiar borate is sodium. Web typical, boron forms 3 covalent bonds. We know that boron is an element whose atomic number is 5. An example is bh3, boron trihydride.
Bf3, Boron Triflouride, Is Another.
Web typically, boron forms 3 covalent bonds. Answer verified 278.7k + views hint: Web boron is highly electronegative, and wants to form compounds with hydrogen atoms. Web typical, boron forms 3 covalent bonds.
Web How Many Single Bonds Can Boron Form?
This is balanced by 5 electrons. The electrons are arranged in shells, with 2 on the first shell and 3 on the. These compounds do not occur in nature. Adhesives, cement, disinfectants, fertilizers, etc.
Typically, Boron Forms 3 Covalent Bonds.
Web see answer (1) best answer. Two of them are core electrons and the remaining 3 are valence electrons. An example is bh3, boron trihydride. Boron commonly makes only three covalent bonds,.
Boron Trifluoride Consists Of A Central Boron Atom With Three Single Bonds To Fluorine Atoms(See Figure Below).
With 3 electrons boron can form 3 single covalent bonds. Boron, b, has an electronic configuration of 1s2 2s2 2p1. A derivative of a borane in which one or more hydrogen atoms have been replaced by. Web boranes are chemical compounds of boron and hydrogen, with the generic formula of b x h y.

/boron-56a12c6f3df78cf772682048.jpg)