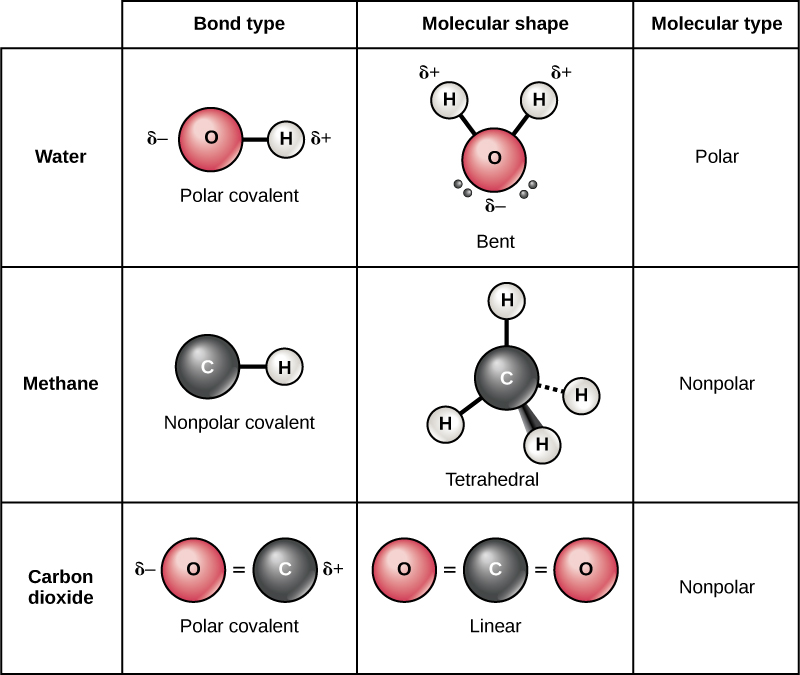
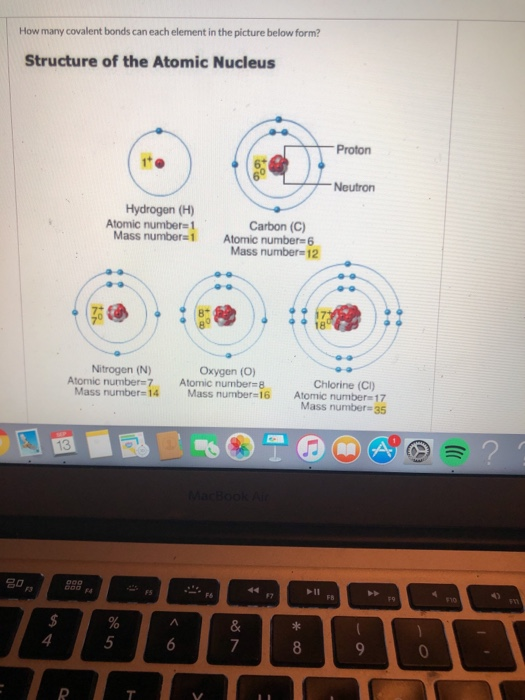
How Many Covalent Bonds Can A Typical Carbon Atom Form
How Many Covalent Bonds Can A Typical Carbon Atom Form - Web well, carbon can form up to four covalent bonds. 45 people found it helpful. The oxygen atom forms two bonds, one with each of two hydrogen atoms;. I'm pretty sure this is chemistry instead of biology haha. Web eduqas carbon use this gcse chemistry study guide to revise the structure of the carbon atom and more. Group 5a form 3 bonds; A bond composed of two electrons, one from each of. Carbon atoms can form a. It has the chemical formula. The bonds may be single, double, or.
Covalent bonds are bonds that are formed between nonmetals. Well, carbon can form up to four covalent bonds. This enables carbon to share four. When it bonds only with hydrogen, it forms compounds called. Four single covalent bonds d. Group 6a form 2 bonds; The methane molecule provides an example: Atoms of carbon can bond with each other or with atoms of other elements. Giant covalent substances contain many atoms joined together by. The absolute values of the electronegativity.
In a covalent bond, two molecules share a couple of electrons. Atoms of carbon can bond with each other or with atoms of other elements. Web well, carbon can form up to four covalent bonds. And group 7a form one bond. [1] the most common form is the single bond: Group 5a form 3 bonds; The oxygen atom forms two bonds, one with each of two hydrogen atoms;. I'm pretty sure this is chemistry instead of biology haha. Carbon atoms can form a. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule.
Carbon to Carbon Single, Double & Triple Bonds Surfguppy
The bonds may be single, double, or. Group 5a form 3 bonds; Web carbon can form four covalent bonds. Two triple covalent bonds c. I'm pretty sure this is chemistry instead of biology haha.
How Many Single Bonds Can Carbon Form fredhughesdesign
Web carbon has four valence electrons, so it can achieve a full outer energy level by forming four covalent bonds. [1] the most common form is the single bond: A bond composed of two electrons, one from each of. Web carbon contains four electrons in its outer shell. Web when the difference is very small or zero, the bond is.
PPT Biochemistry PowerPoint Presentation, free download ID89333
Covalent bonds are bonds that are formed between nonmetals. Atoms of carbon can bond with each other or with atoms of other elements. Two triple covalent bonds c. And group 7a form one bond. Web well, carbon can form up to four covalent bonds.
Carbon — Role and Importance to Life Expii
Web carbon can form four covalent bonds. Atoms of carbon can bond with each other or with atoms of other elements. Web each carbon atom forms four covalent bonds. I'm pretty sure this is chemistry instead of biology haha. Covalent bonds are bonds that are formed between nonmetals.
Atoms, Isotopes, Ions, and Molecules The Building Blocks · Biology
This enables carbon to share four. The absolute values of the electronegativity. Well, carbon can form up to four covalent bonds. Two triple covalent bonds c. The methane molecule provides an example:
Solved How many covalent bonds can each element in the
When it is large, the bond is polar covalent or ionic. Two triple covalent bonds c. Web well, carbon can form up to four covalent bonds. The simplest organic carbon molecule is methane. The absolute values of the electronegativity.
2.2 Bonding and Lattices Physical Geology
Web each carbon atom forms four covalent bonds. Web eduqas carbon use this gcse chemistry study guide to revise the structure of the carbon atom and more. Web when the difference is very small or zero, the bond is covalent and nonpolar. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet.
2.2 Chemical Bonds Anatomy & Physiology
The absolute values of the electronegativity. There is no 4 bond formed between carbon because of the carbon electron orbitals. [1] the most common form is the single bond: When it bonds only with hydrogen, it forms compounds called. Giant covalent substances contain many atoms joined together by.
11 Types of scientific changes with examples
Web why carbon does not form 4 bonds with itself? The simplest organic carbon molecule is methane. Giant covalent substances contain many atoms joined together by. And group 7a form one bond. 45 people found it helpful.
Covalent Bonding (Biology) — Definition & Role Expii
Web carbon has four valence electrons, so it can achieve a full outer energy level by forming four covalent bonds. Web each carbon atom forms four covalent bonds. Covalent bonds are bonds that are formed between nonmetals. Since it has 4 valence. Group 5a form 3 bonds;
Four Single Covalent Bonds D.
Covalent bonds are bonds that are formed between nonmetals. Web because carbon has four electrons in its valence (outer) shell, it can form four covalent bonds with other atoms or molecules. Carbon atoms can form a. The methane molecule provides an example:
There Is No 4 Bond Formed Between Carbon Because Of The Carbon Electron Orbitals.
Therefore, it can form four covalent bonds with other atoms or molecules. And group 7a form one bond. Web carbon can form four covalent bonds. This enables carbon to share four.
Web Carbon Contains Four Electrons In Its Outer Shell.
Web in order to form a covalent bond, each element has to share one unpaired electron. Web carbon has four valence electrons, so it can achieve a full outer energy level by forming four covalent bonds. Web eduqas carbon use this gcse chemistry study guide to revise the structure of the carbon atom and more. Atoms of carbon can bond with each other or with atoms of other elements.
But Anyways, A Typical Carbon Atom Can Form Up To 4 Covalent.
One double covalent bond b. Well, carbon can form up to four covalent bonds. Since it has 4 valence. Web why carbon does not form 4 bonds with itself?