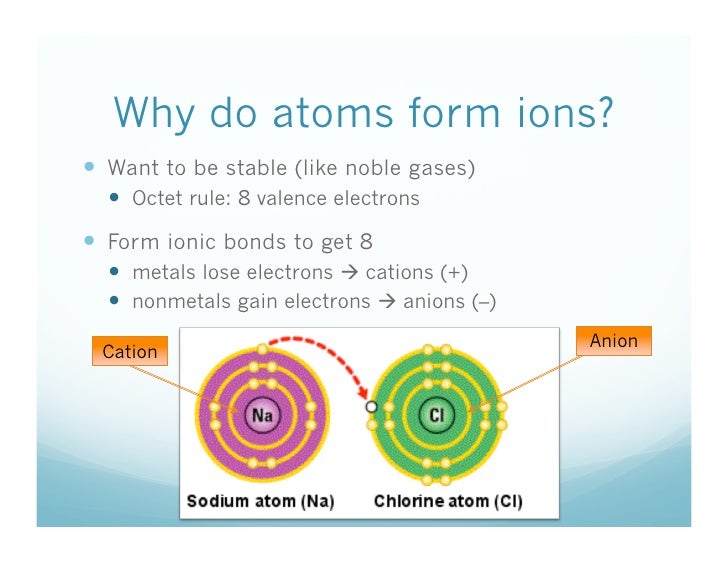
Metals Lose Electrons And Form
Metals Lose Electrons And Form - Web metals tend to lose electrons and form positively charged ions called cations. Web magnesium’s position in the periodic table (group 2) tells us that it is a metal. Web metal atoms lose electrons from their outer shell when they form ions: Web ions form when atoms lose or gain electrons. Are oxidized) when they undergo chemical reactions they. Web metal atoms lose electrons to nonmetal atoms because metals typically have relatively low ionization energies. This creates oppositely charged ions : The reactivity series of metals is a chart showing metals in order of decreasing. Metals at the bottom of a group lose electrons more easily than. To obtain a full outer shell:
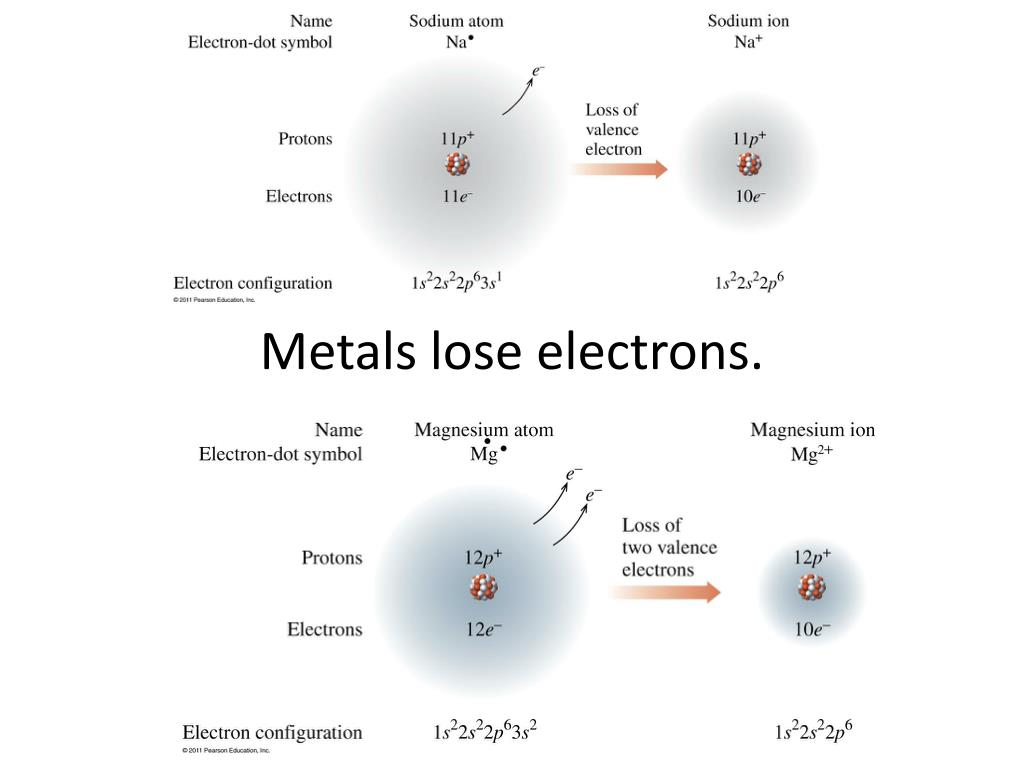
Metals form positive ions (cations). A magnesium atom must lose two electrons to have the same. Web metals are electropositive elements that generally form basic or amphoteric oxides with oxygen. Web 1 answer anor277 nov 12, 2015 from a modern atomic perspective, the metal stops losing ions when it reaches a reasonably stable electronic configuration. Web metal atoms lose electrons from their outer shell when they form ions: In this process, they either lose or gain electrons or. Metals at the bottom of a group lose electrons more easily than. Metal atoms lose electrons to form positive ions (. To obtain a full outer shell: Web metals tend to lose electrons and form positively charged ions called cations.
Web metals are electropositive elements that generally form basic or amphoteric oxides with oxygen. To obtain a full outer shell: Web when metals react with other substances, the metal atoms lose electrons to form positive ions. Web 1 answer anor277 nov 12, 2015 from a modern atomic perspective, the metal stops losing ions when it reaches a reasonably stable electronic configuration. Metals at the bottom of a group lose electrons more easily than. Metal atoms lose electrons to form positive ions (. Are oxidized) when they undergo chemical reactions they. To illustrate, an atom of an. Metals form positive ions (cations). Web metals tend to lose electrons to form positive ions because, for metals to gain a full outer shell, they need to lose electrons.
Chem matters ch6_ionic_bond
Web metals tend to lose electrons and form positively charged ions called cations. Metal atoms lose electrons to form positive ions (. Metals tend to have low ionization energies, and typically lose electrons (i.e. Web ions form when atoms lose or gain electrons. Web 1 answer anor277 nov 12, 2015 from a modern atomic perspective, the metal stops losing ions.
PPT Metals, Nonmetals, and Movement of Electrons PowerPoint
A magnesium atom must lose two electrons to have the same. Metals at the bottom of a group lose electrons more easily than. Do metals tend to loose electrons to form. Web metals tend to lose electrons and form positively charged ions called cations. Web when metals react with other substances, the metal atoms lose electrons to form positive ions.
The3Chemiteers Trends On the Periodic Table
A magnesium atom must lose two electrons to have the same. Web metals tend to lose electrons to form positive ions because, for metals to gain a full outer shell, they need to lose electrons. To obtain a full outer shell: The ions formed are positive, because they have more protons than electrons the ions formed have full outer. To.
Ions Predict Charge Stone Cold Chemistry Talk Ions Predict Charge
Web 1 answer anor277 nov 12, 2015 from a modern atomic perspective, the metal stops losing ions when it reaches a reasonably stable electronic configuration. Metals tend to have low ionization energies, and typically lose electrons (i.e. Web metal atoms lose electrons from their outer shell when they form ions: A magnesium atom must lose two electrons to have the.
Write My Paper For Me how to write ions
To illustrate, an atom of an. Web metals tend to lose electrons to form positive ions because, for metals to gain a full outer shell, they need to lose electrons. The reactivity series of metals is a chart showing metals in order of decreasing. Do metals tend to loose electrons to form. Web when metals react with other substances, the.
4.7 Ions Losing & Gaining Electrons YouTube
The reactivity series of metals is a chart showing metals in order of decreasing. To illustrate, an atom of an. Metals form positive ions (cations). Web metals tend to lose electrons and form positively charged ions called cations. Metal atoms lose electrons to form positive ions (.
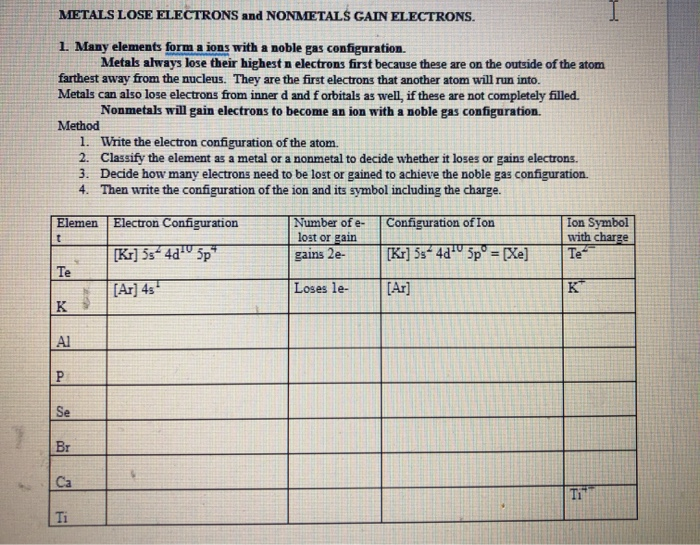
Solved METALS LOSE ELECTRONS and NONMETALS GAIN ELECTRONS.
Metals tend to have low ionization energies, and typically lose electrons (i.e. Web metals tend to lose electrons to form positive ions because, for metals to gain a full outer shell, they need to lose electrons. Web metals tend to lose electrons and form positively charged ions called cations. This creates oppositely charged ions : Web ions form when atoms.
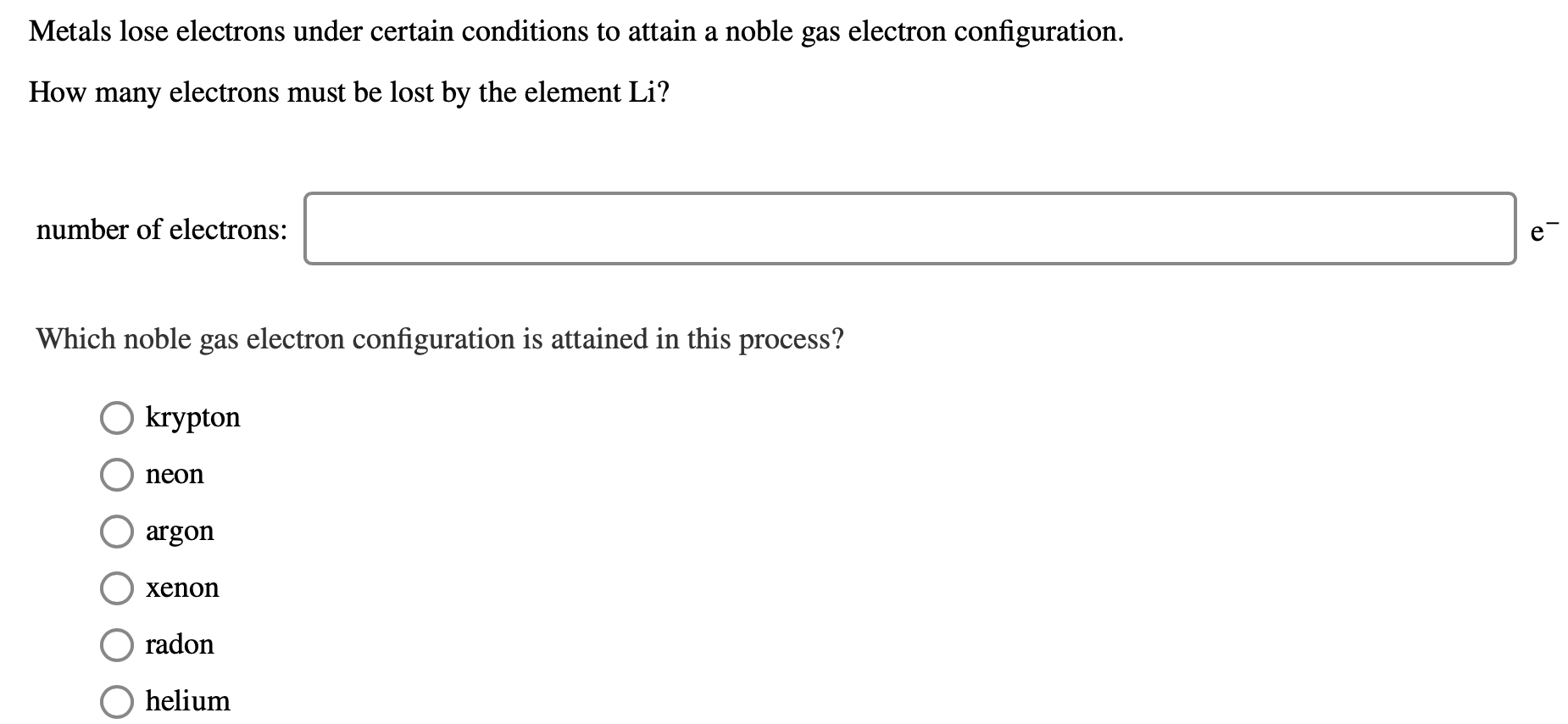
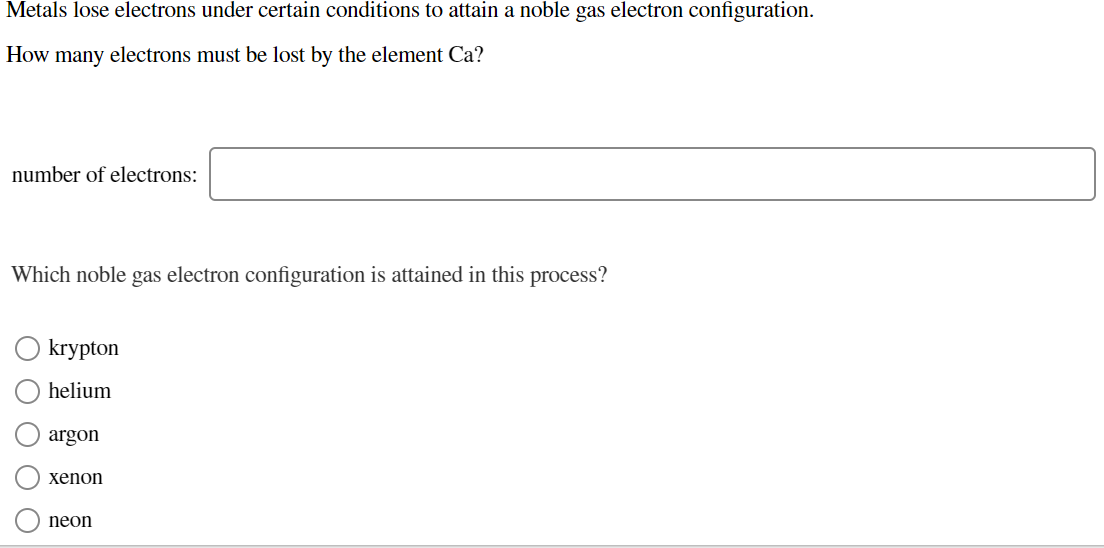
Solved Metals lose electrons under certain conditions to
Do metals tend to loose electrons to form. A magnesium atom must lose two electrons to have the same. Web ions form when atoms lose or gain electrons. Web 1 answer anor277 nov 12, 2015 from a modern atomic perspective, the metal stops losing ions when it reaches a reasonably stable electronic configuration. In this process, they either lose or.
PPT Chemistry 120 PowerPoint Presentation, free download ID6788469
The ions formed are positive, because they have more protons than electrons the ions formed have full outer. To obtain a full outer shell: Web solution the correct option is d they form ionic compounds elements try to become stable by attaining inert gas configuration. In this process, they either lose or gain electrons or. Web metal atoms lose electrons.
Answered Metals lose electrons under certain… bartleby
Metals form positive ions (cations). Web metals are electropositive elements that generally form basic or amphoteric oxides with oxygen. To obtain a full outer shell: Metal atoms lose electrons to form positive ions (. To illustrate, an atom of an.
Web Ions Form When Atoms Lose Or Gain Electrons.
Web metal atoms lose electrons to nonmetal atoms because metals typically have relatively low ionization energies. Web magnesium’s position in the periodic table (group 2) tells us that it is a metal. Web metals tend to lose electrons and form positively charged ions called cations. Are oxidized) when they undergo chemical reactions they.
Web Metals Tend To Lose Electrons To Form Positive Ions Because, For Metals To Gain A Full Outer Shell, They Need To Lose Electrons.
The reactivity series of metals is a chart showing metals in order of decreasing. To illustrate, an atom of an. To obtain a full outer shell: Web 1 answer anor277 nov 12, 2015 from a modern atomic perspective, the metal stops losing ions when it reaches a reasonably stable electronic configuration.
In This Process, They Either Lose Or Gain Electrons Or.
Do metals tend to loose electrons to form. This creates oppositely charged ions : Metals form positive ions (cations). Metals at the bottom of a group lose electrons more easily than.
To Obtain A Full Outer Shell:
A magnesium atom must lose two electrons to have the same. Web metals are electropositive elements that generally form basic or amphoteric oxides with oxygen. Metal atoms lose electrons to form positive ions (. Web when metals react with other substances, the metal atoms lose electrons to form positive ions.