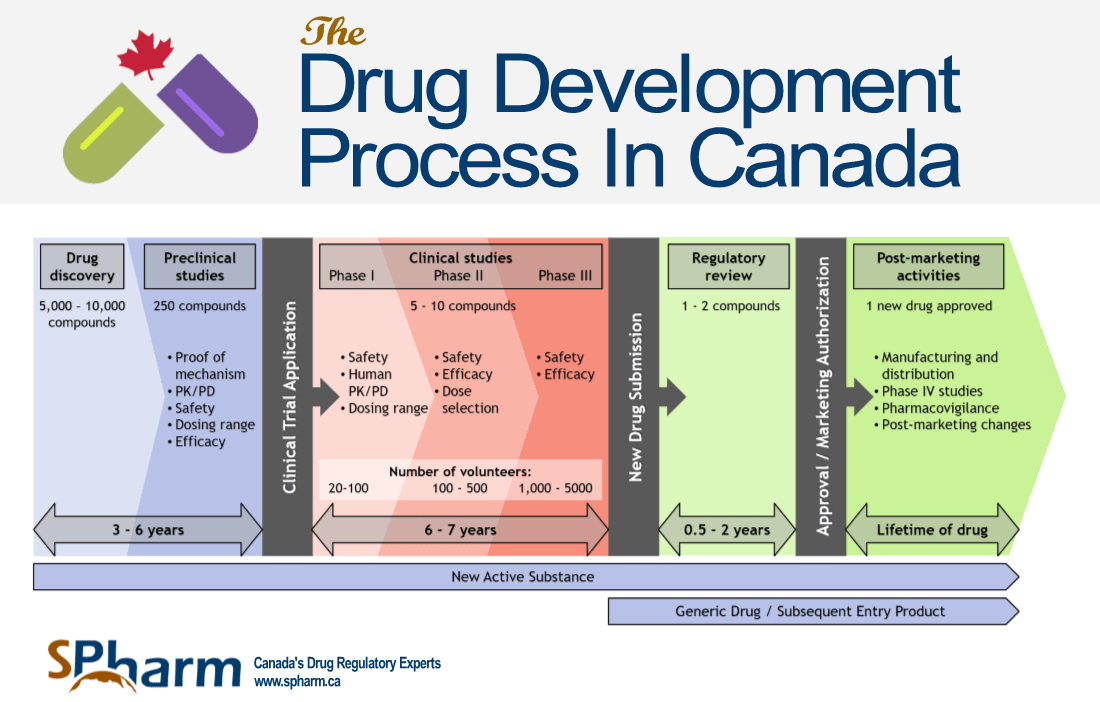
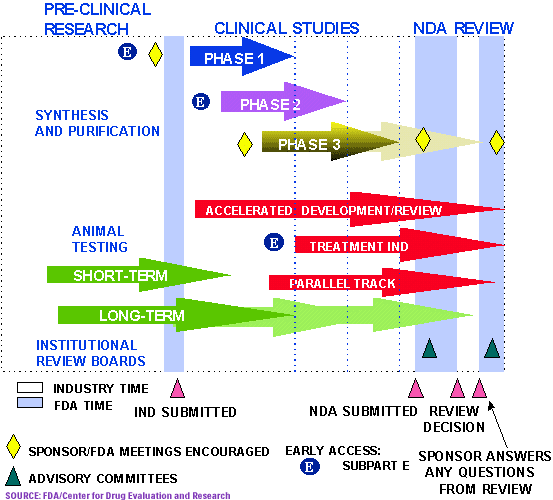
Pharmaceutical Approval Calendar
Pharmaceutical Approval Calendar - Web pharmacists met with lawmakers at the state capitol to discuss an emergency rule to help them deal with pharmacy benefit managers and the associated. See more catalyst data with a free or premium account. See table 1 for new approvals in. Web annual numbers of new molecular entities (nmes) and biologics license applications (blas) approved by the fda’s cder. Unlock more data with a free account. All medicines must be authorised before they can be marketed and made available to patients. In the european union (eu), there are two main routes. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline that covers. Track key readouts and fda decision dates in biotech and pharma. Web the table below is a running list of cder’s novel drugs approvals for 2024.
Web benzinga's fda calendar shows historical fda data, upcoming dates that companies will be impacted by the fda and ranges of dates. Web the table below is a running list of cder’s novel drugs approvals for 2024. Stay updated on biotech earnings. See table 1 for new approvals in. Web below is a listing of new molecular entities and new therapeutic biological products approved by cder and organized by calendar year. Track key readouts and fda decision dates in biotech and pharma. Web this page lists the timetables for the submission, start and finish dates of procedures, as well as other interim dates and milestones that occur during the various procedures. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline that covers. Only showing catalysts expected in next 14 days. See more catalyst data with a free or premium account.
Stay updated on biotech earnings. Web fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. Web pharmacists met with lawmakers at the state capitol to discuss an emergency rule to help them deal with pharmacy benefit managers and the associated. See more catalyst data with a free or premium account. Web a quick and dirty guide to upcoming fda approval dates for biotech drugs. Web our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. All medicines must be authorised before they can be marketed and made available to patients. Web this page lists the timetables for the submission, start and finish dates of procedures, as well as other interim dates and milestones that occur during the various procedures. Vanda pharmaceuticals has failed to persuade the fda to approve its. Food and drug administration has declined to approve vanda pharmaceuticals' drug to treat stomach paralysis symptoms, the company said on.
Calendar of Events Pharmaceutical Society of Singapore
In the european union (eu), there are two main routes. Web get daily updates on important fda approval, pdufa dates, and fda advisory committee meetings with rttnews fda calendar & upcoming approvals. Stay updated on biotech earnings. The pdufa date refers to the date the food and. Web below is a listing of new molecular entities and new therapeutic biological.
Red Pill on the Calendar. Schedule Medication Stock Photo Image of
Web below is a listing of new molecular entities and new therapeutic biological products approved by cder and organized by calendar year. See more catalyst data with a free or premium account. Web get daily updates on important fda approval, pdufa dates, and fda advisory committee meetings with rttnews fda calendar & upcoming approvals. Web benzinga's fda calendar shows historical.
Pharmaceutical Contracting Asbury, NJ Calendar
Web the table below is a running list of cder’s novel drugs approvals for 2024. Web this page lists the timetables for the submission, start and finish dates of procedures, as well as other interim dates and milestones that occur during the various procedures. Web get daily updates on important fda approval, pdufa dates, and fda advisory committee meetings with.
Pharmaceutical & Cosmetic Calendar 2019 by New Media B2B Issuu
Unlock more data with a free account. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline that covers. Web our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. The pdufa.
16+ Drug Approval Process Timeline, New Ideas
Stay updated on biotech earnings. Web fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. Vanda pharmaceuticals has failed to persuade the fda to approve its. Web evoke pharma’s commitment to quality and innovation continues to drive its mission to provide patients with reliable and effective treatments for gastroparesis. Web.
Fda Approval Dates For Drugs at Wanda Fisher blog
Web reuters.com is your online source for the latest news stories and current events, ensuring our readers up to date with any breaking news developments Only showing catalysts expected in next 14 days. Web fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. The pdufa date refers to the date.
Medicines. Pink pharmaceutical capsules with tablets are in the
Vanda pharmaceuticals has failed to persuade the fda to approve its. Food and drug administration has declined to approve vanda pharmaceuticals' drug to treat stomach paralysis symptoms, the company said on. Web our pdufa calendar includes future pdufa dates for biotech companies, as well as advisory committee meeting dates. Web annual numbers of new molecular entities (nmes) and biologics license.
FDA Calendar FDA Tracker
The pdufa date refers to the date the food and. Food and drug administration has declined to approve vanda pharmaceuticals' drug to treat stomach paralysis symptoms, the company said on. All medicines must be authorised before they can be marketed and made available to patients. In the european union (eu), there are two main routes. Web fda calendar for drug.
Updated The Global Pharmaceutical Packaging & Labelling Countdown
Web get daily updates on important fda approval, pdufa dates, and fda advisory committee meetings with rttnews fda calendar & upcoming approvals. See table 1 for new approvals in. Web below is a listing of new molecular entities and new therapeutic biological products approved by cder and organized by calendar year. Web below is a listing of new molecular entities.
40 Great Medication Schedule Templates (+Medication Calendars)
Only showing catalysts expected in next 14 days. Food and drug administration has declined to approve vanda pharmaceuticals' drug to treat stomach paralysis symptoms, the company said on. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline that covers. Web annual numbers of new molecular entities (nmes).
Web The Table Below Is A Running List Of Cder’s Novel Drugs Approvals For 2024.
Web a quick and dirty guide to upcoming fda approval dates for biotech drugs. Vanda pharmaceuticals has failed to persuade the fda to approve its. Web our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. Web our free fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including clinical trials, regulatory events, and.
See More Catalyst Data With A Free Or Premium Account.
Web evoke pharma’s commitment to quality and innovation continues to drive its mission to provide patients with reliable and effective treatments for gastroparesis. Web reuters.com is your online source for the latest news stories and current events, ensuring our readers up to date with any breaking news developments Only showing catalysts expected in next 14 days. All medicines must be authorised before they can be marketed and made available to patients.
Web Our Pdufa Calendar Includes Future Pdufa Dates For Biotech Companies, As Well As Advisory Committee Meeting Dates.
Showing 1 to 23 of 23 entries. Web home drugs development & approval process | drugs how drugs are developed and approved drug and biologic approval and ind activity reports nda. Web below is a listing of new molecular entities and new therapeutic biological products approved by cder and organized by calendar year. Web annual numbers of new molecular entities (nmes) and biologics license applications (blas) approved by the fda’s cder.
Track Key Readouts And Fda Decision Dates In Biotech And Pharma.
Web pharmacists met with lawmakers at the state capitol to discuss an emergency rule to help them deal with pharmacy benefit managers and the associated. Stay updated on biotech earnings. Unlock more data with a free account. In the european union (eu), there are two main routes.