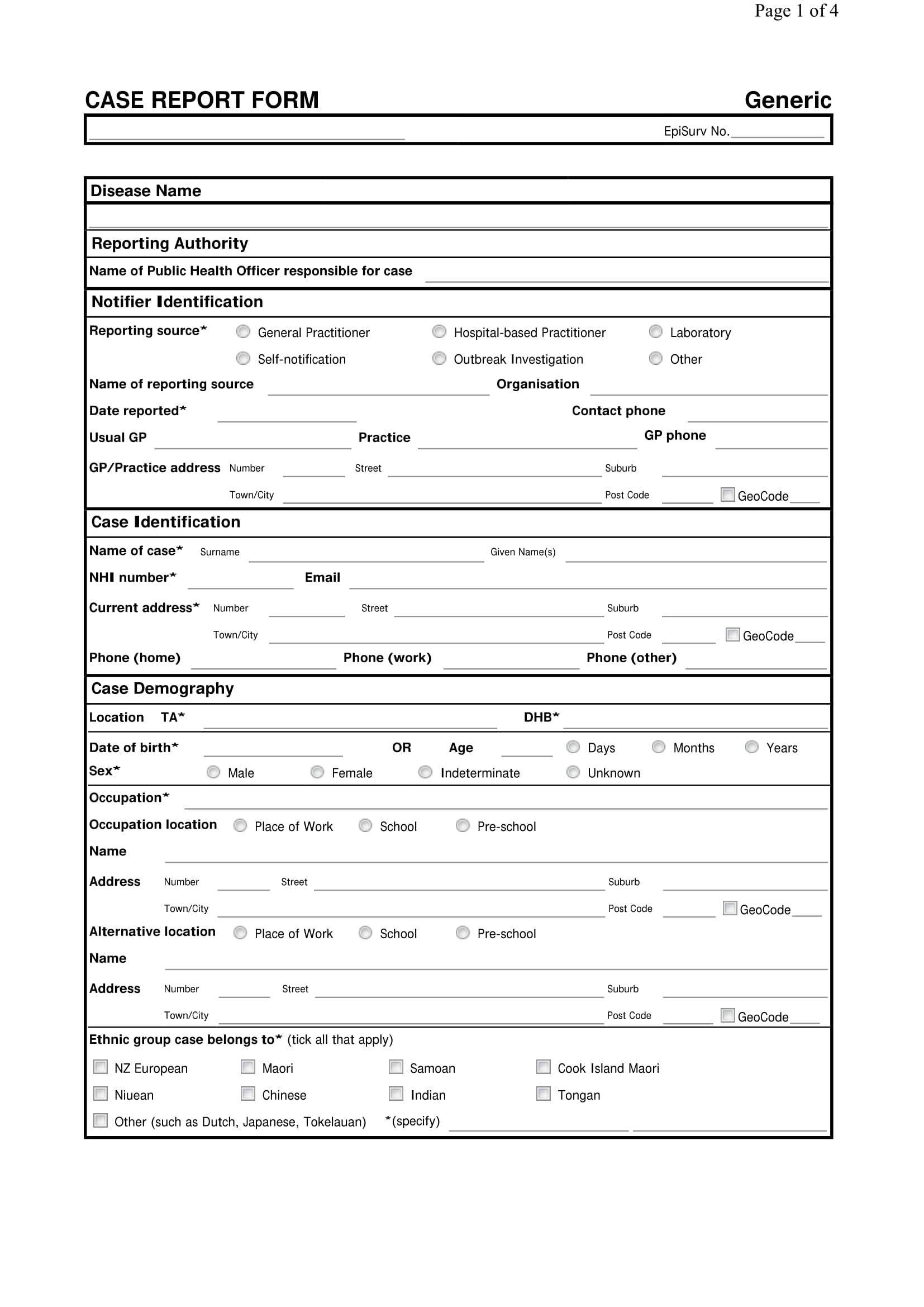
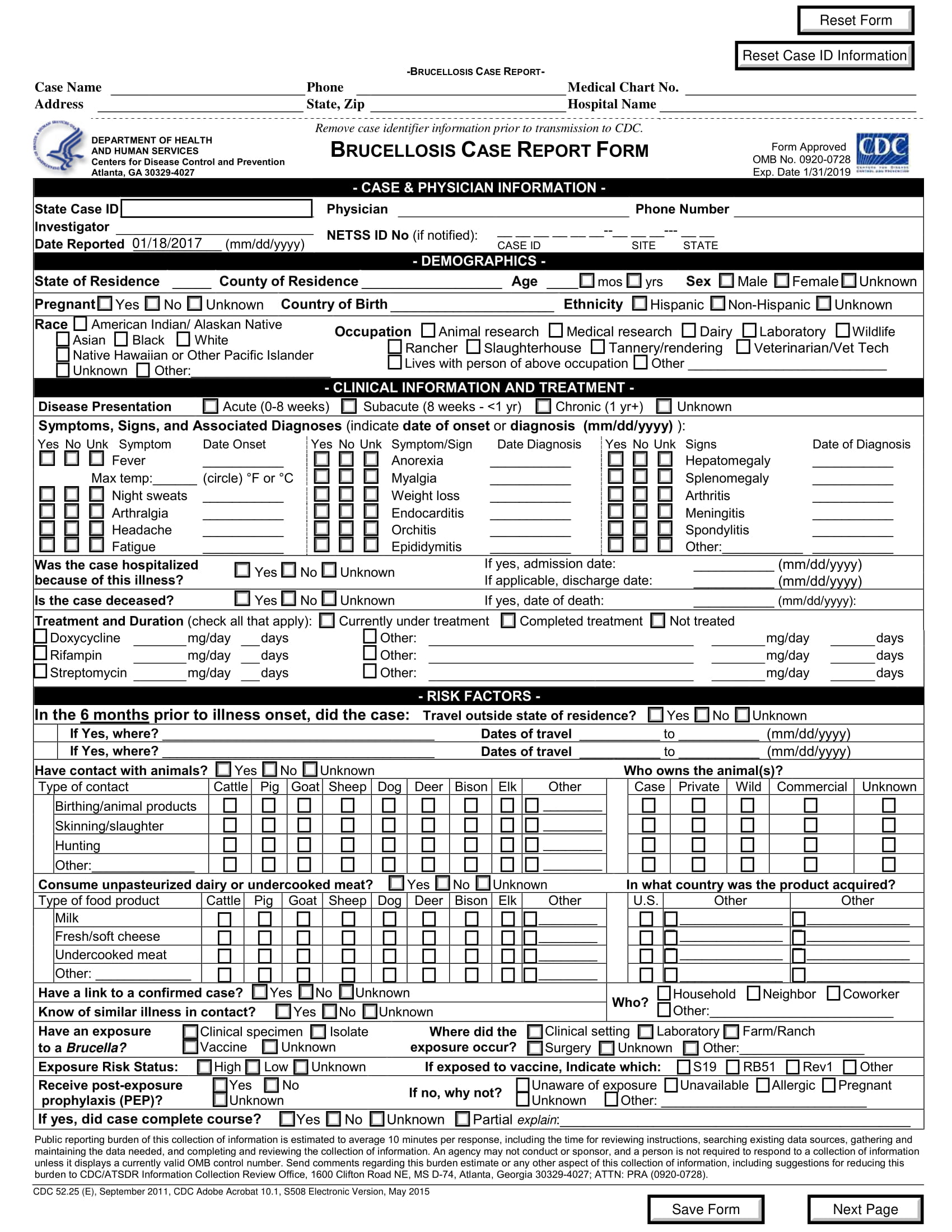
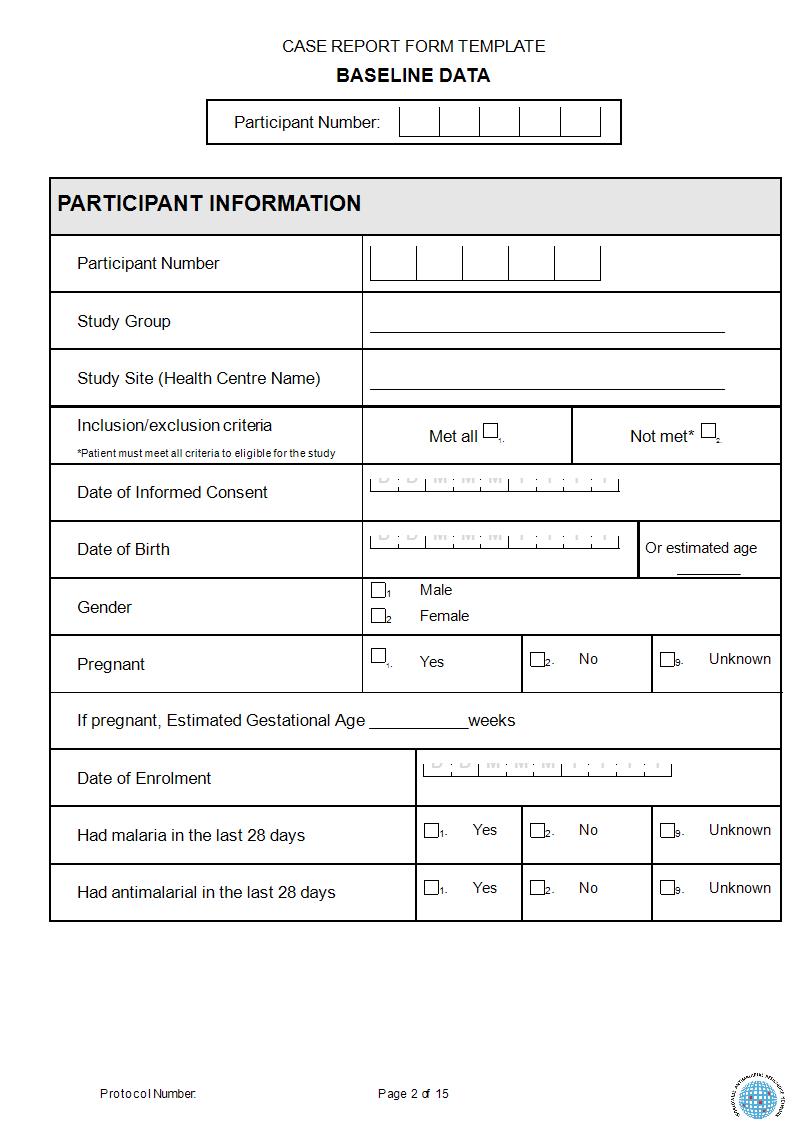
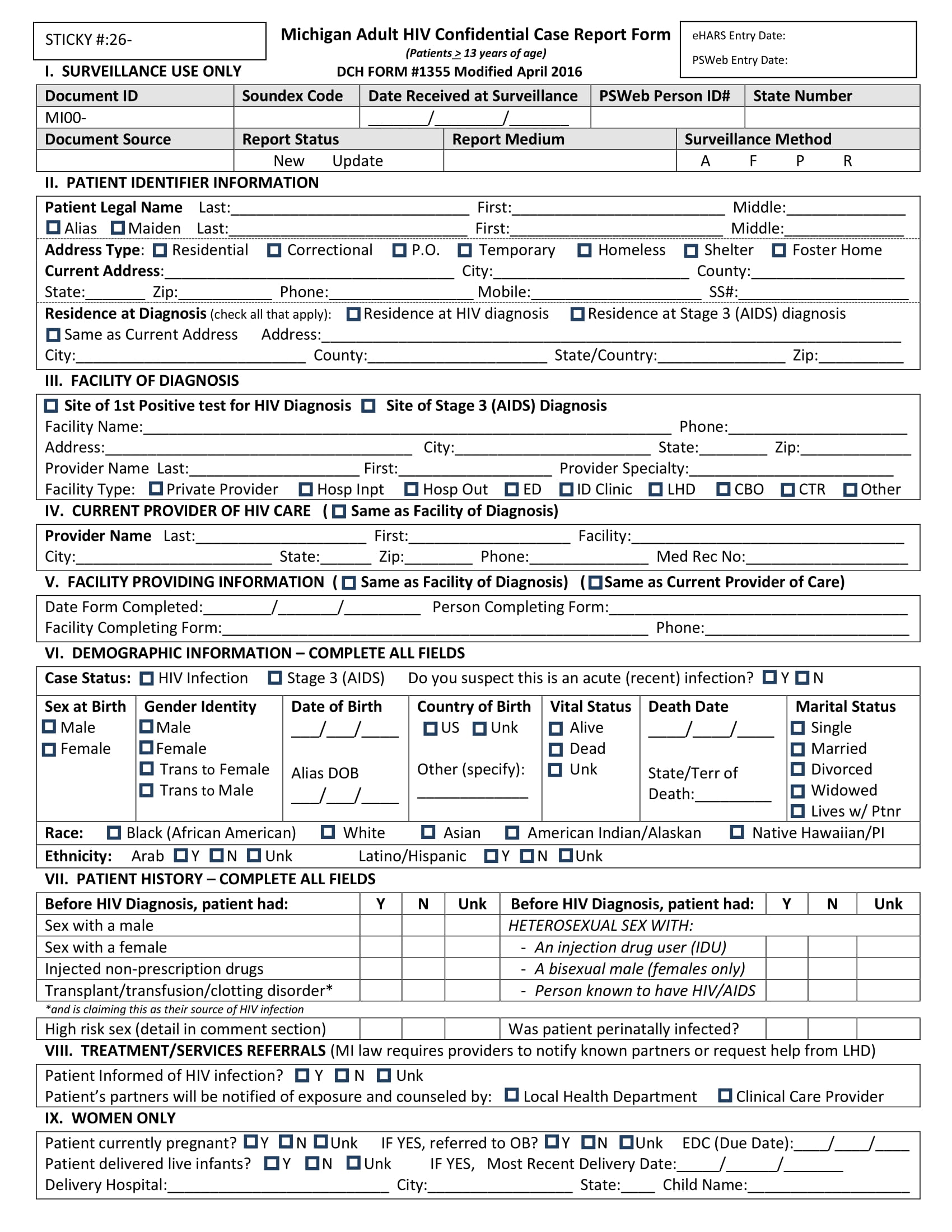
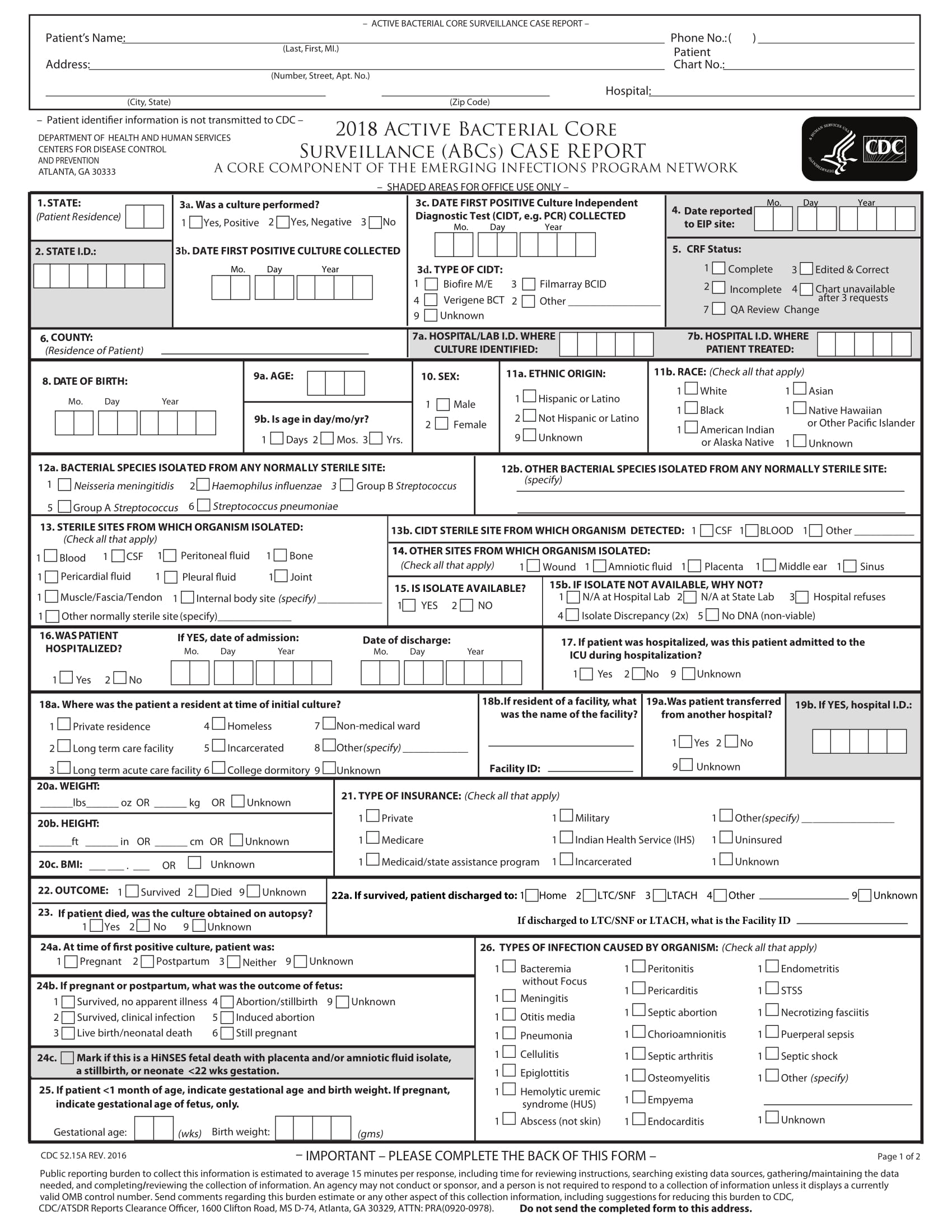
What Is A Case Report Form
What Is A Case Report Form - Web a case report form (crf) is a document that is created and used in clinical trials to capture standardized study data from each patient to answer the research question. Case reports usually describe an. It should be study protocol driven, robust in content and have material to collect the study specific. The crf is used by the study sponsor to. Web a case report is a document that defines and details a certain symptom, illness, injury, diagnosis, treatment or recovery of a patient. A medical case report, also known as a case study, is a detailed description of a clinical encounter with a patient. Web a case report form is a standardized questionnaire that is used during a clinical trial to collect data from each patient. Web a case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each participant. A case report is referred to the clinical researches that are documented from the different case trials. Web what is a case report form?
Web in medical terms, crf stands for case report form. Web what is a case report form? Web a case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each participant. Us homeowners are sitting on roughly. The most important aspect of a case. Web the case report is a specific type of research design that reports on an aspect of the management of one or two patients. The report is usually based on the actual. Case report form (crf) is a specialized document in clinical research. It should be study protocol driven, robust in content and have material to collect the study specific. A medical case report, also known as a case study, is a detailed description of a clinical encounter with a patient.
A written account of a decided case giving the main points of the argument on each side, the court's findings, and the decision reached. Web the case report is a specific type of research design that reports on an aspect of the management of one or two patients. Web case report forms (crfs) are arguably the most important documentation in a clinical trial since they are the last point of data entry, which ultimately influences the outcome of a. Web a case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each participant. Case reports usually describe an. Web this resource will help you develop a learning network, and form a study group. A case report is referred to the clinical researches that are documented from the different case trials. Web what is a case report form? The crf is used by the study sponsor to. The report is usually based on the actual.
10+ Case Report Templates PDF, Word, Pages Free & Premium Templates
A written account of a decided case giving the main points of the argument on each side, the court's findings, and the decision reached. With this case report form, sponsors and medical. The crf is used by the study sponsor to. It is the first piece of research writing in the health. Web what is a case report?
FREE 15+ Case Report Forms in PDF MS Word
Web what is a case report? Web what is a case report form? A crf is a set of documents that collects data and information from a clinical trial. The report is usually based on the actual. Web this resource will help you develop a learning network, and form a study group.
FREE 15+ Case Report Forms in PDF MS Word
A case report is referred to the clinical researches that are documented from the different case trials. Case report form (crf) is a specialized document in clinical research. Web what is a case report form? The most important aspect of a case. It should be study protocol driven, robust in content and have material to collect the study.
FREE 15+ Case Report Forms in PDF MS Word
Web a case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each participant. Case report form (crf) is a specialized document in clinical research. A medical case report, also known as a case study, is a detailed description of a clinical encounter with a.
FREE 15+ Case Report Forms in PDF MS Word
Short articles and blog posts this resource provides tips and. Web eastman, who would also author trump’s supreme court brief in the texas case, spent much of december leaning on state legislators to declare their elections. Us homeowners are sitting on roughly. Web this resource will help you develop a learning network, and form a study group. Web the case.
FREE 15+ Case Report Forms in PDF MS Word
Web a case report form is a standardized questionnaire that is used during a clinical trial to collect data from each patient. The most important aspect of a case. Web what is a case report form? Short articles and blog posts this resource provides tips and. Web the case report is a specific type of research design that reports on.
Case Report Form Template Clinical Trials
Web the housing market has grown less affordable over the last 4 months, even as prices just saw their biggest annual decline in a decade. Short articles and blog posts this resource provides tips and. Web in medical terms, crf stands for case report form. With this case report form, sponsors and medical. Web agent x revealed − and he's.
10+ Case Report Templates PDF, Word, Pages Free & Premium Templates
Web agent x revealed − and he's a democrat. Web a case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each participant. Web 13 hours agoa federal grand jury has indicted former president donald trump in special counsel jack smith’s investigation into efforts to.
FREE 15+ Case Report Forms in PDF MS Word
Web a case report form (crf) is a document that is created and used in clinical trials to capture standardized study data from each patient to answer the research question. The most important aspect of a case. A medical case report, also known as a case study, is a detailed description of a clinical encounter with a patient. With this.
Embalming Case Report Fill Online, Printable, Fillable, Blank pdfFiller
The crf is used by the study sponsor to. It is the first piece of research writing in the health. Web a case report is a document that defines and details a certain symptom, illness, injury, diagnosis, treatment or recovery of a patient. Web case report forms (crfs) are arguably the most important documentation in a clinical trial since they.
The Crf Is Used By The Study Sponsor To.
Web in medical terms, crf stands for case report form. Web eastman, who would also author trump’s supreme court brief in the texas case, spent much of december leaning on state legislators to declare their elections. Web a case report form is a standardized questionnaire that is used during a clinical trial to collect data from each patient. The report is usually based on the actual.
Web What Is A Case Report?
Web what is a case report form? Web case report forms (crfs) are arguably the most important documentation in a clinical trial since they are the last point of data entry, which ultimately influences the outcome of a. Web what is a case report form? A crf is a set of documents that collects data and information from a clinical trial.
It Should Be Study Protocol Driven, Robust In Content And Have Material To Collect The Study.
With this case report form, sponsors and medical. Case reports usually describe an. Web the case report is a research design where an unexpected or novel occurrence is described in a detailed report of findings, clinical course, and. It should be study protocol driven, robust in content and have material to collect the study specific.
Web A Case Report Is A Document That Defines And Details A Certain Symptom, Illness, Injury, Diagnosis, Treatment Or Recovery Of A Patient.
A case report is referred to the clinical researches that are documented from the different case trials. It is the first piece of research writing in the health. Web a case report form (crf) is an electronic or paper document which is used in a clinical trial to record the protocol and required information about each participant. Case report form (crf) is a specialized document in clinical research.