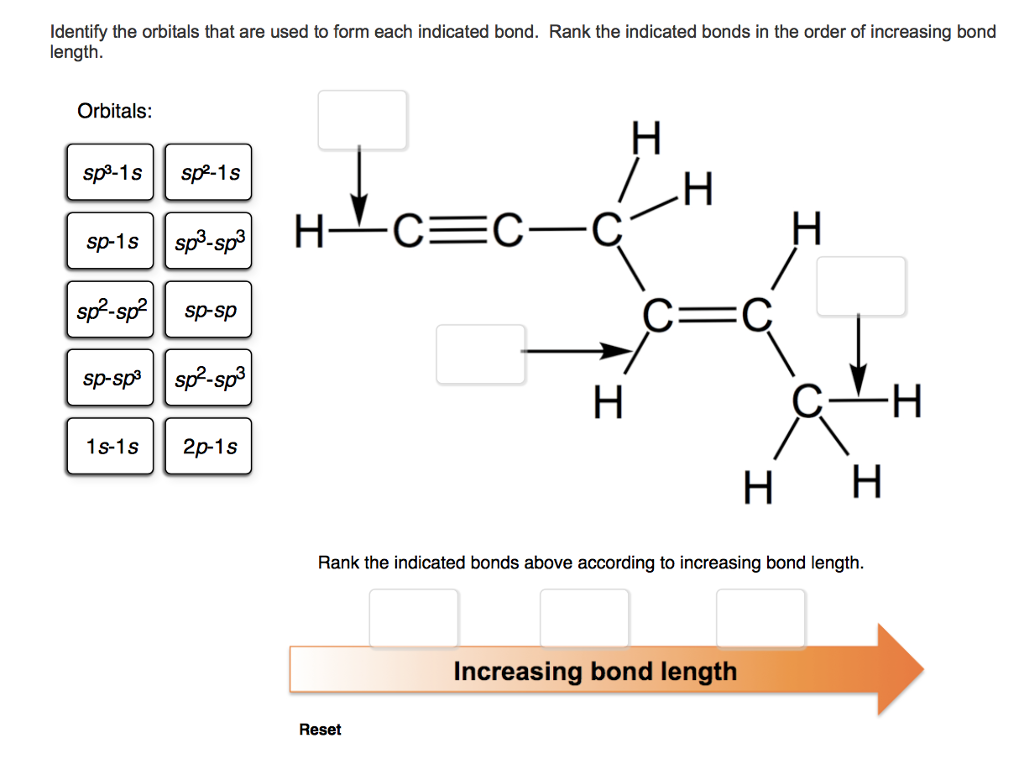
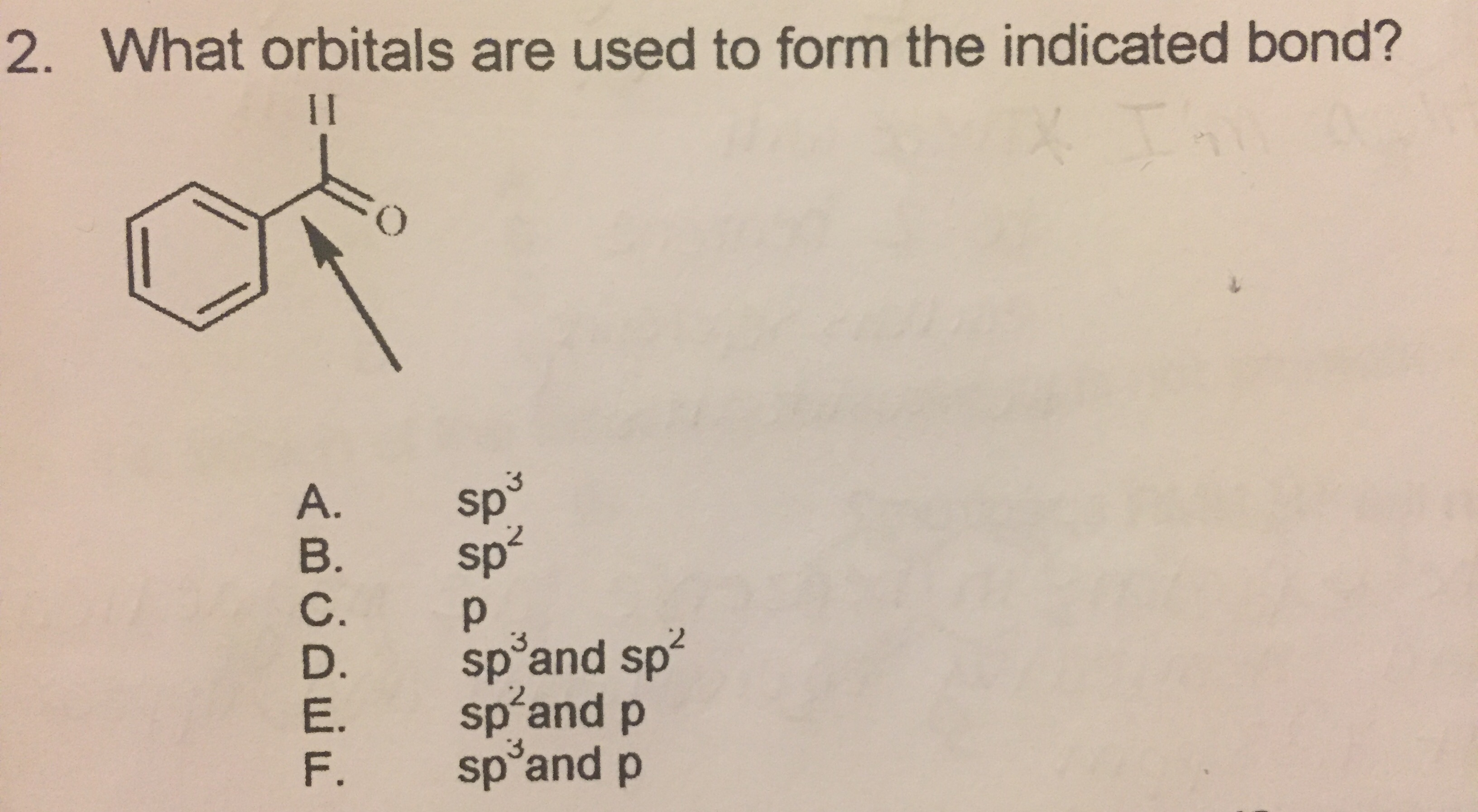
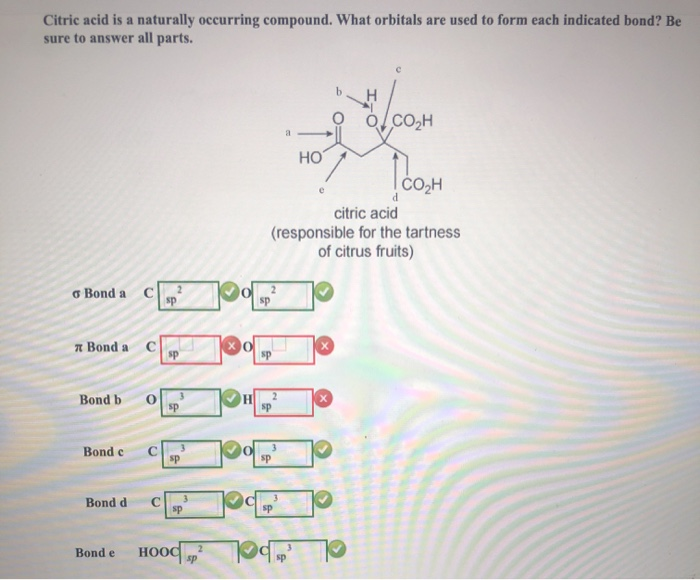
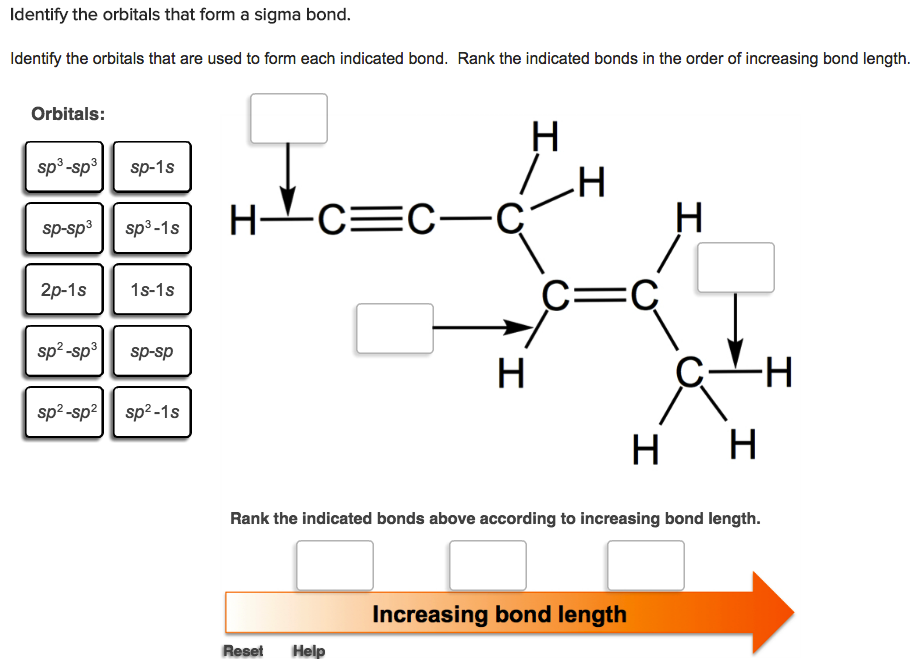
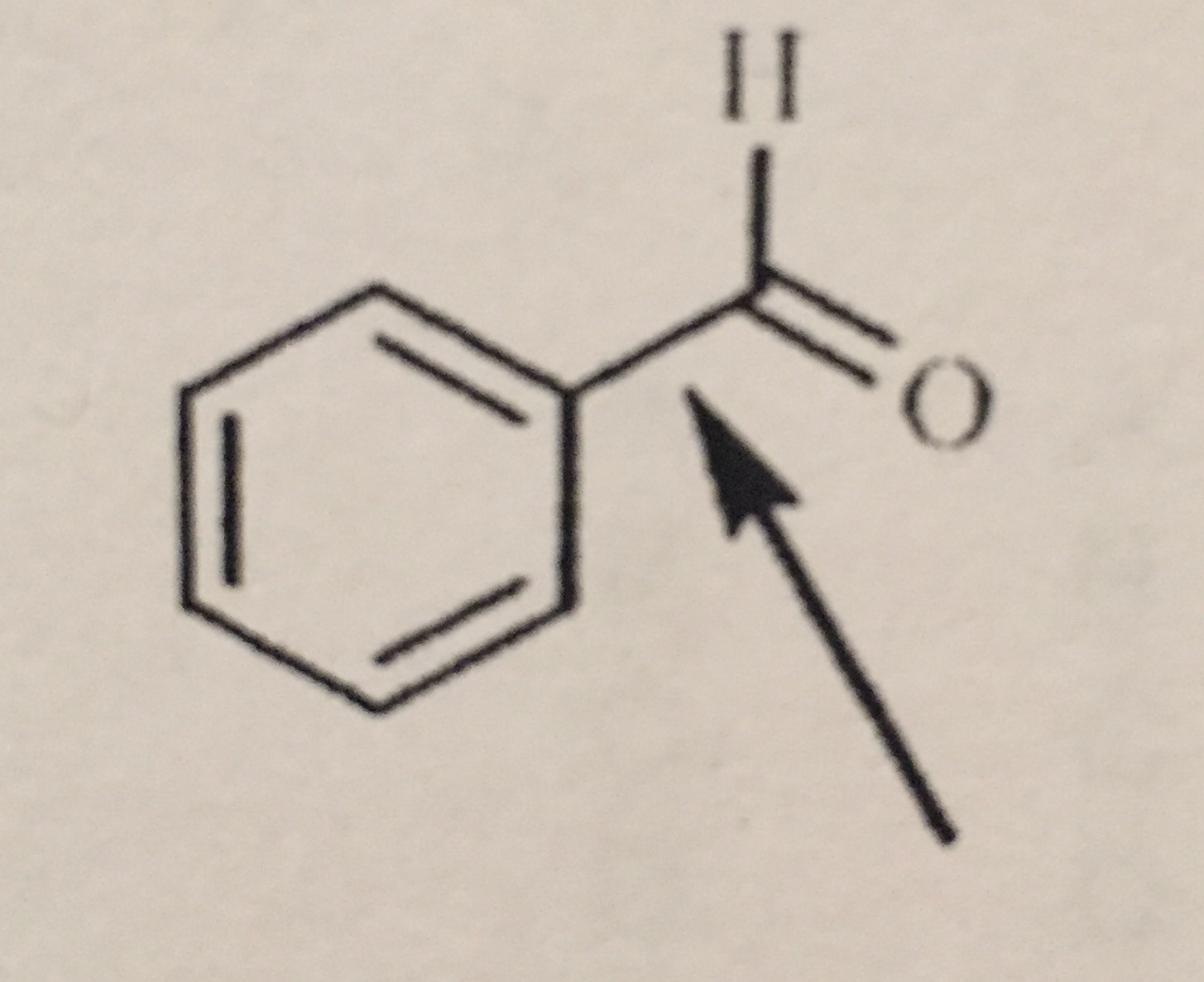
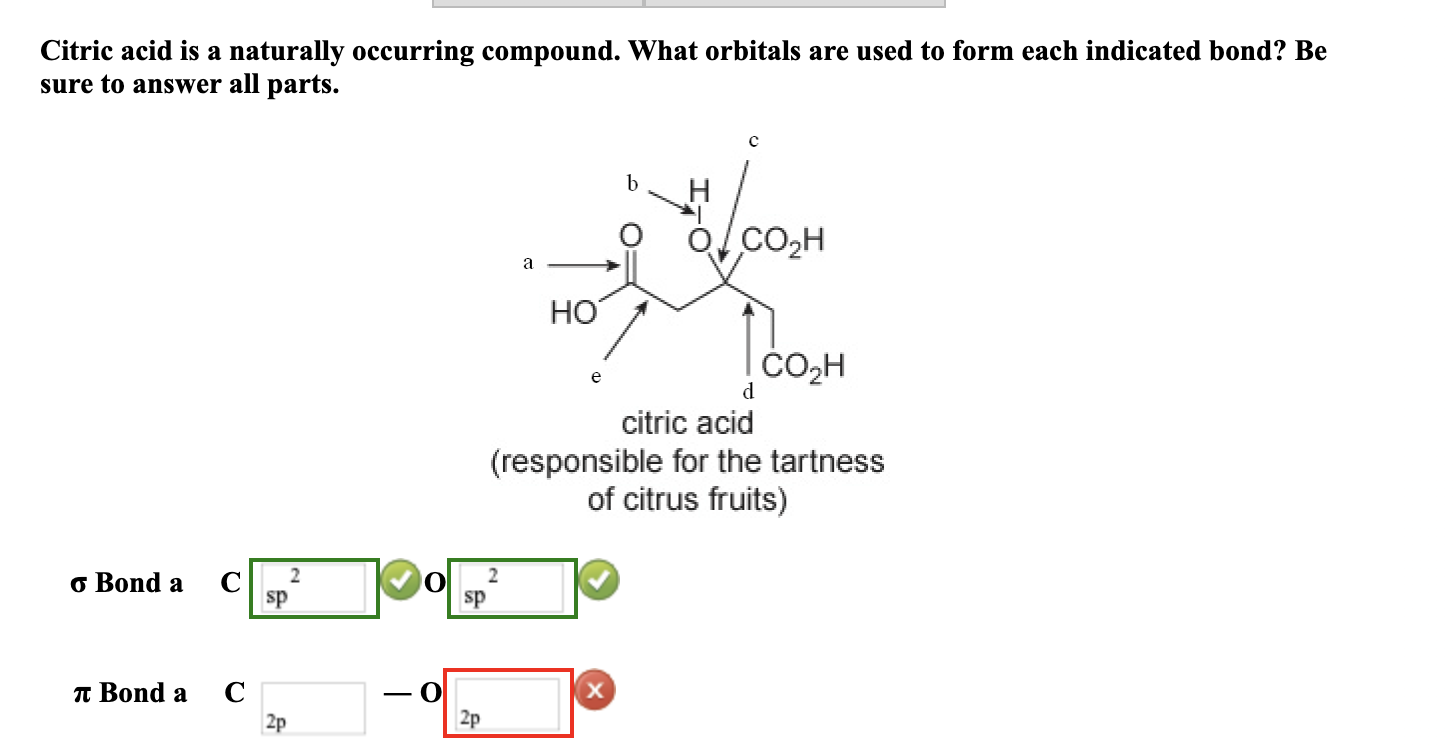
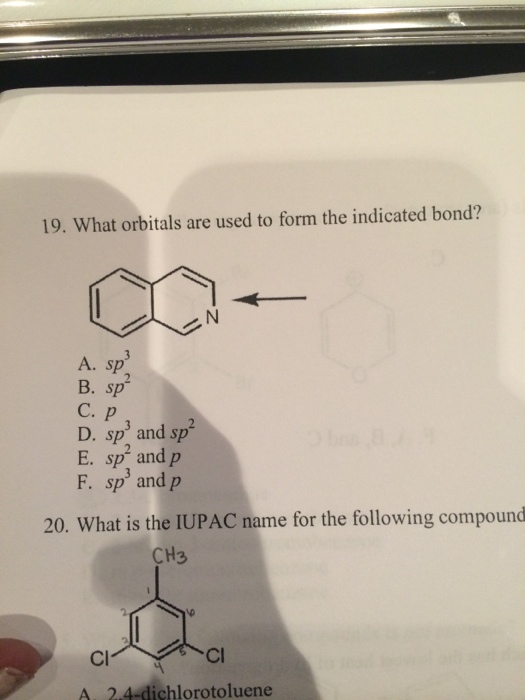
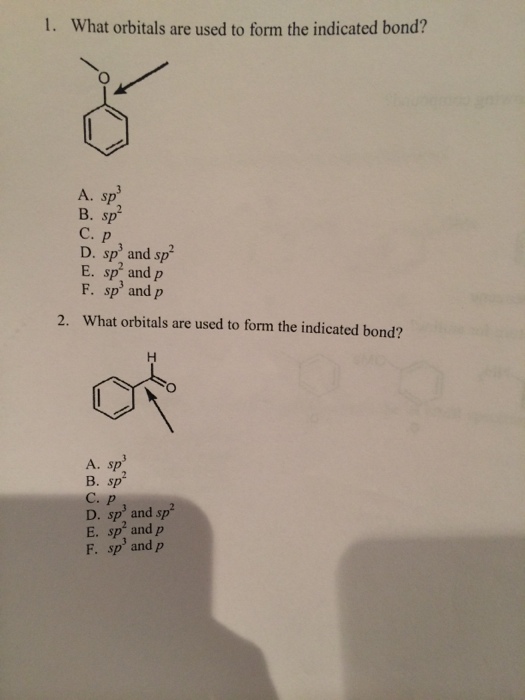What Orbitals Are Used To Form The Indicated Bond
What Orbitals Are Used To Form The Indicated Bond - Web organic chemistry short answer question: In the following sections, we shall discuss the common types of hybrid orbitals. Considers electrons delocalized throughout the entire molecule. Citric acid is a naturally occurring compound. Web what hybrid orbitals are used by the carbon atoms in hc≡ch? Ch 3 och 3 answer a. Citric acid is a naturally occurring compound. Web this problem has been solved! What orbitals are used to form each indicated bond? What orbitals are used to form.
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Be sure to answer all parts. What orbitals are used to form. Unhybridized orbitals overlap to form π bonds. Web chemistry questions and answers. If there is more than one bond, you have to indicate the orbital's for each individual bob to. What orbitals are used to form each bond in the following molecules? In the following sections, we shall discuss the common types of hybrid orbitals. Web considers bonds as localized between one pair of atoms. Web in this model, bonds are considered to form from the overlapping of two atomic orbitals on different atoms, each orbital containing a single electron.
Web in this model, bonds are considered to form from the overlapping of two atomic orbitals on different atoms, each orbital containing a single electron. What orbitals are used to form each indicated bond? Web what hybrid orbitals are used by the carbon atoms in hc≡ch? Determine what orbital's are being used to form each of the bonds. The bond between the two. Ch 3 och 3 answer a. Considers electrons delocalized throughout the entire molecule. Citric acid is a naturally occurring compound. The orbital utilized for the formation o. Web organic chemistry short answer question:
Solved What orbitals are used to form the indicated bond.
Web considers bonds as localized between one pair of atoms. Be sure to answer all parts. Web what hybrid orbitals are used by the carbon atoms in hc≡ch? Citric acid is a naturally occurring compound. If there is more than one bond, you have to indicate the orbital's for each individual bob to.
OneClass what orbitals are used to form the indicated bond?
Citric acid is a naturally occurring compound. Citric acid is a naturally occurring compound. Unhybridized orbitals overlap to form π bonds. What orbitals are used to form the indicated bond. Web what hybrid orbitals are used by the carbon atoms in hc≡ch?
OneClass what orbitals are used to form the indicated bond?
Web chemistry questions and answers. Ch 3 och 3 answer a. The bond between the two. Citric acid is a naturally occurring compound. What orbitals are used to form each indicated bond?
Solved Identify the orbitals that are used to form each
Determine what orbital's are being used to form each of the bonds. If there is more than one bond, you have to indicate the orbital's for each individual bob to. Unhybridized orbitals overlap to form π bonds. Citric acid is a naturally occurring compound. What orbitals are used to form each bond in the following molecules?
Solved What orbitals are used to form the indicated bond?
Be sure to answer all parts. Web what hybrid orbitals are used by the carbon atoms in hc≡ch? Web we can use hybrid orbitals, which are mathematical combinations of some or all of the valence atomic orbitals, to describe the electron density around covalently bonded. Web this problem has been solved! What orbitals are used to form each indicated bond?
Solved Citric acid is a naturally occurring compound. What
Be sure to answer all parts. Considers electrons delocalized throughout the entire molecule. What orbitals are used to form. 100% (2 ratings) answer step 1 sigma bond is f. Citric acid is a naturally occurring compound.
Chemistry Archive June 12, 2016
Web considers bonds as localized between one pair of atoms. The orbital utilized for the formation o. In looking at simple inorganic. What orbitals are used to form each indicated bond? Web chemistry questions and answers.
Solved What orbitals are used to form the indicated bonds?
What orbitals are used to form each indicated bond? The orbital utilized for the formation o. In the following sections, we shall discuss the common types of hybrid orbitals. Creates bonds from overlap of atomic orbitals ( s, p, d. What orbitals are used to form each indicated bond?
Solved Citric acid is a naturally occurring compound. What
What orbitals are used to form each of the indicated bonds?. What orbitals are used to form the indicated bond. Web chemistry questions and answers. What orbitals are used to form each indicated bond? Web organic chemistry short answer question:
Solved What orbitals are used to form the indicated bond?
( ch 3) 3 b c. Web we can use hybrid orbitals, which are mathematical combinations of some or all of the valence atomic orbitals, to describe the electron density around covalently bonded. Citric acid is a naturally occurring compound. Web considers bonds as localized between one pair of atoms. The bond between the two.
Web Chemistry Questions And Answers.
In the following sections, we shall discuss the common types of hybrid orbitals. Unhybridized orbitals overlap to form π bonds. Determine what orbital's are being used to form each of the bonds. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
In Looking At Simple Inorganic.
The bond between the two. Web organic chemistry short answer question: What orbitals are used to form each bond in the following molecules? What orbitals are used to form.
Creates Bonds From Overlap Of Atomic Orbitals ( S, P, D.
What orbitals are used to form the indicated bond. ( ch 3) 3 b c. Web chemistry questions and answers. Web we can use hybrid orbitals, which are mathematical combinations of some or all of the valence atomic orbitals, to describe the electron density around covalently bonded.
This Molecule Is Linear, And It Consists Of 3 Sigma,.
Be sure to answer all parts. Web hybrid orbitals overlap to form σ bonds. What orbitals are used to form each indicated bond? What orbitals are used to form each of the indicated bonds?.