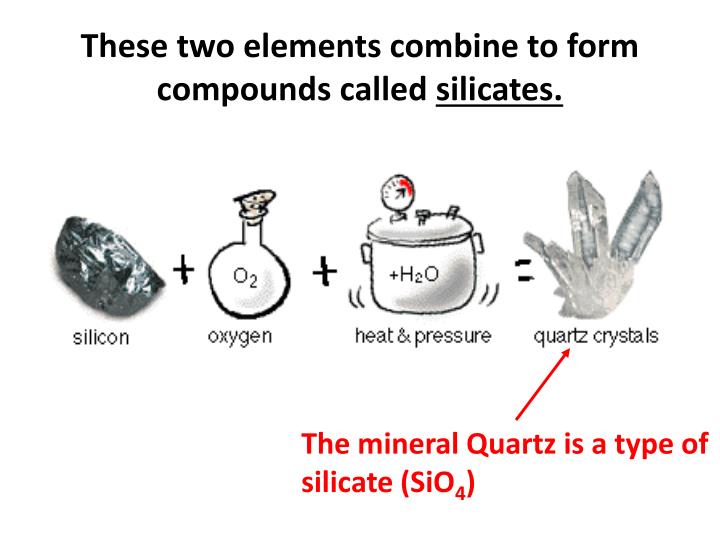
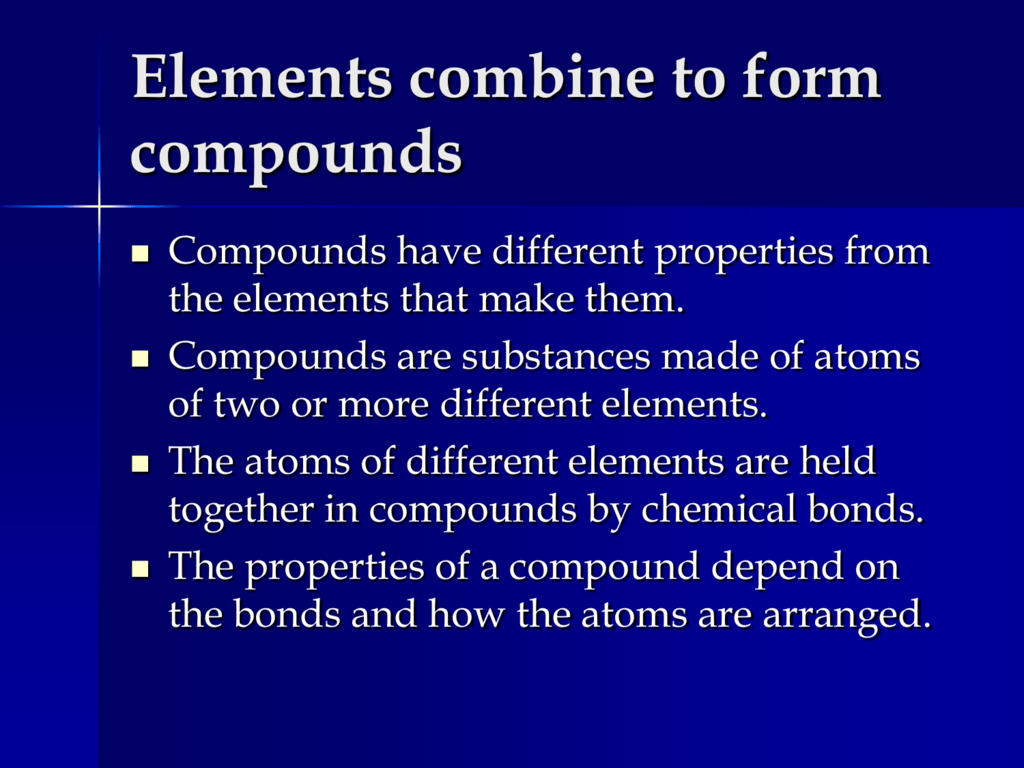
When Elements Combine To Form Compounds:
When Elements Combine To Form Compounds: - A compound consists of two or more types of elements held together by. \ [\ce {a} + \ce {b} \rightarrow \ce {ab}\nonumber \]. Metals often react with nonmetals to form ionic compounds. These combinations take place because almost all. Web to understand how elements are combined to form compounds, it is necessary to understand the structure of atoms. Web almost all the elements combine to form compounds, although the reactivity may vary from element to element. The general form of a combination reaction is: Web ions compounds elements combine to form chemical compounds that are often divided into two categories. For example, hydrogen and oxygen atoms combine in a ratio of 2:1 to form the. Web the elements carbon and hydrogen combine to form many different compounds.
Web the third part says compounds are combinations of two or more different types of atoms. \ [\ce {a} + \ce {b} \rightarrow \ce {ab}\nonumber \]. Web almost all the elements combine to form compounds, although the reactivity may vary from element to element. Web atoms of different elements may combine with each other in a fixed, simple, whole number ratios to form compound atoms. A compound consists of two or more types of elements held together by. These data help justify an atomic view. One of the simplest is called methane, in which there are always four times as many hydrogen atoms as carbon atoms. The other type of synthesis reaction happens. Atoms cannot be created or destroyed. Web ions compounds elements combine to form chemical compounds that are often divided into two categories.
For example, hydrogen and oxygen atoms combine in a ratio of 2:1 to form the. Metals often react with nonmetals to form ionic compounds. Web chemical bonds link elements together to form more complex molecules called compounds. Atoms consist mainly of electrically charged particles. These data help justify an atomic view. Web term 1 / 14 chemical bonds click the card to flip 👆 definition 1 / 14 what compounds form through, which are links between two or more atoms that hold the atoms together click. Web ions compounds elements combine to form chemical compounds that are often divided into two categories. The fourth part of the theory states that a chemical reaction is a rearrangement of atoms. Atoms cannot be created or destroyed. Web atoms of different elements may combine with each other in a fixed, simple, whole number ratios to form compound atoms.
Elements combine to form compounds.
Web when two or more elements combine to form a compound, their masses in that compound are in a fixed and definite ratio. Metals often react with nonmetals to form ionic compounds. The fourth part of the theory states that a chemical reaction is a rearrangement of atoms. Atoms consist mainly of electrically charged particles. Atoms cannot be created or.
PPT Minerals PowerPoint Presentation ID2047598
Web when elements combine to form compounds: The other type of synthesis reaction happens. Their properties do not change b). The fourth part of the theory states that a chemical reaction is a rearrangement of atoms. Atoms of same element can combine in more than.
What Is a Compound in Chemistry? Definition and Examples
Web chemical bonds link elements together to form more complex molecules called compounds. Atoms consist mainly of electrically charged particles. Methane is a pure substance because it always has the same. Web yes, when elements combine to form compounds, their individual properties are lost. Web combination reactions can also be called synthesis reactions.
Elements combine to form compounds YouTube
In comparison, elements retain some (or many) of their property when they are mixed to. Web combination reactions can also be called synthesis reactions. A compound consists of two or more types of elements held together by. The general form of a combination reaction is: Web ions compounds elements combine to form chemical compounds that are often divided into two.
PPT Elements combine to form compounds. PowerPoint Presentation, free
A compound consists of two or more types of elements held together by. Their properties do not change b). Web yes, when elements combine to form compounds, their individual properties are lost. Web when two or more elements combine to form a compound, their masses in that compound are in a fixed and definite ratio. Web ions compounds elements combine.
PPT Elements combine to form compounds. PowerPoint Presentation, free
Web combination reactions can also be called synthesis reactions. These data help justify an atomic view. Web chemical bonds link elements together to form more complex molecules called compounds. \ [\ce {a} + \ce {b} \rightarrow \ce {ab}\nonumber \]. Web atoms of different elements may combine with each other in a fixed, simple, whole number ratios to form compound atoms.
Elements combine to form compounds
Methane is a pure substance because it always has the same. Web the elements carbon and hydrogen combine to form many different compounds. Web when two or more elements combine to form a compound, their masses in that compound are in a fixed and definite ratio. Web almost all the elements combine to form compounds, although the reactivity may vary.
How to Combine Elements to Form Compounds Sciencing
The fourth part of the theory states that a chemical reaction is a rearrangement of atoms. Web law of multiple proportions, statement that when two elements combine with each other to form more than one compound, the weights of one element that combine with a fixed. Web when two or more elements combine to form a compound, their masses in.
Elements & compounds 1 Ionic Bonding.avi YouTube
Web the elements carbon and hydrogen combine to form many different compounds. Web atoms of the same element are similar in shape and mass, but differ from the atoms of other elements. Web yes, when elements combine to form compounds, their individual properties are lost. Web chemical bonds link elements together to form more complex molecules called compounds. Web term.
How to Combine Elements to Form Compounds Sciencing
Web the third part says compounds are combinations of two or more different types of atoms. Atoms cannot be created or destroyed. A compound consists of two or more types of elements held together by. In comparison, elements retain some (or many) of their property when they are mixed to. Web term 1 / 14 chemical bonds click the card.
Web Atoms Of The Same Element Are Similar In Shape And Mass, But Differ From The Atoms Of Other Elements.
A compound consists of two or more types of elements held together by. Web when elements combine to form compounds: Web term 1 / 14 chemical bonds click the card to flip 👆 definition 1 / 14 what compounds form through, which are links between two or more atoms that hold the atoms together click. These data help justify an atomic view.
Their Properties Change Completely C).
Metals often react with nonmetals to form ionic compounds. One of the simplest is called methane, in which there are always four times as many hydrogen atoms as carbon atoms. Web when two or more elements combine to form a compound, their masses in that compound are in a fixed and definite ratio. \ [\ce {a} + \ce {b} \rightarrow \ce {ab}\nonumber \].
The Other Type Of Synthesis Reaction Happens.
Web the elements carbon and hydrogen combine to form many different compounds. Their properties do not change b). Atoms cannot be created or destroyed. Web to understand how elements are combined to form compounds, it is necessary to understand the structure of atoms.
Web Almost All The Elements Combine To Form Compounds, Although The Reactivity May Vary From Element To Element.
The fourth part of the theory states that a chemical reaction is a rearrangement of atoms. Web law of multiple proportions, statement that when two elements combine with each other to form more than one compound, the weights of one element that combine with a fixed. Web yes, when elements combine to form compounds, their individual properties are lost. Web ions compounds elements combine to form chemical compounds that are often divided into two categories.