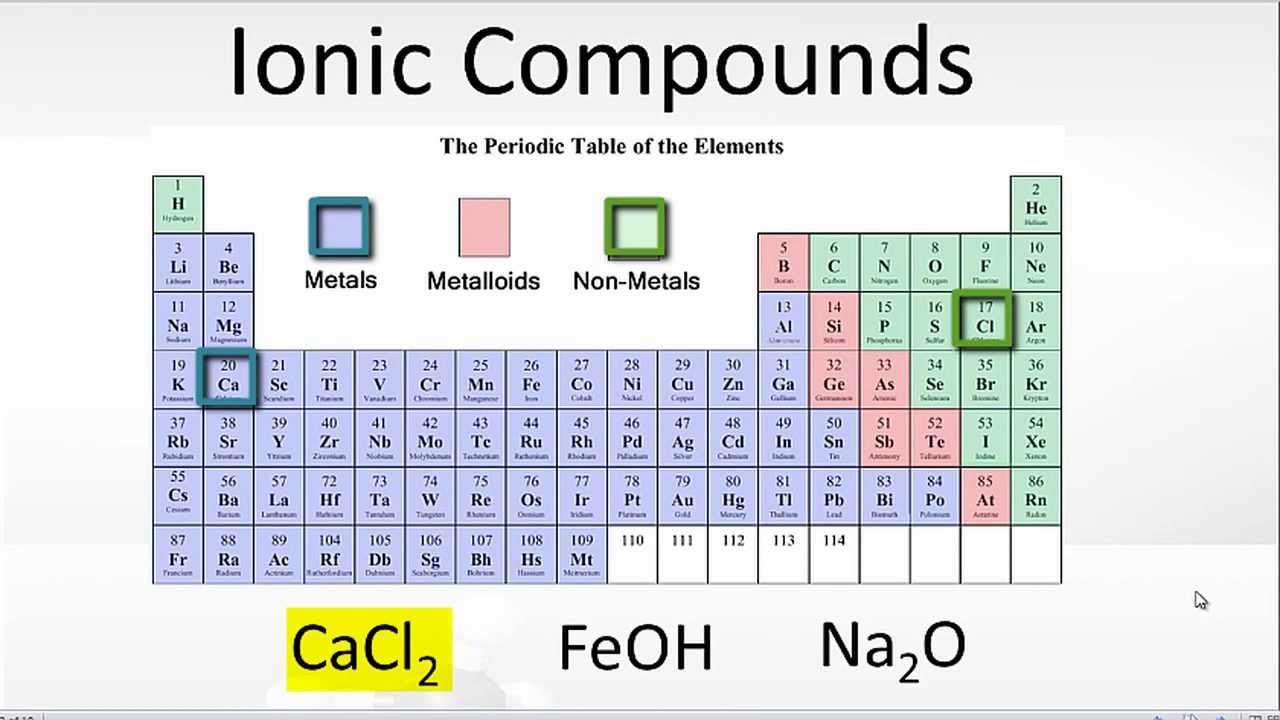
Which Elements Form Ionic Bonds
Which Elements Form Ionic Bonds - Web ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Web use the periodic table to describe which elements form ionic bonds and which elements form covalent bonds. Web ionic bonding is the complete transfer of valence electron(s) between atoms. Ionic bonds covalent bonds nacl. 1) for example, consider na and cl. Metals and nonmetals, electrons transferred. Which of the following elements is likely to form ionic bonds? Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Web chemistry chemistry questions and answers (a) use the periodic table to describe which elements form ionic bonds, and which elements form covalent bonds. (select all that apply.) chlorine (cl) calcium (ca) helium (he) sodium (na) fluorine (f) carbon (c), potassium is a _____.
One that tends to lose electrons and one that tends to gain them suppose an oxygen atom gains two electrons to become an oxygen ion. 1) for example, consider na and cl. Web which elements form ionic bonds? Metals and metals, electrons freely moving. Web barium (ba) phosphorus (p) radon (rn) lithium (li) (b) list five examples of compounds with ionic bonds. One has been shown for you. Web ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Web between which type of elements do ionic bonds occur, and how do electrons act within the bond? Web study with quizlet and memorize flashcards containing terms like review the discussion of electronegativity and the discussion of ionic bonds in section 2.2.
(b) list five examples of compounds with ionic bonds. (select all that apply.) chlorine (cl) calcium (ca) helium (he) sodium (na) fluorine (f) carbon (c), potassium is a _____. It is a type of chemical bond that generates two oppositely charged ions. Metals and nonmetals, electrons transferred. Which of the following elements is likely to form ionic bonds? Web study with quizlet and memorize flashcards containing terms like review the discussion of electronegativity and the discussion of ionic bonds in section 2.2. The atom that loses the electrons becomes a positively charged ion (. Metals and metals, electrons freely moving. Name at least five ionic bonds and five covalent bonds and enter them in the table below. Web ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
Web ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond that generates two oppositely charged ions. Web ionic bonding is the complete transfer of valence electron(s) between atoms. Web use the periodic table to describe which elements form ionic bonds and which elements form covalent bonds. Web study with quizlet.
Ionic bonding Wikipedia
It is a type of chemical bond that generates two oppositely charged ions. 3) last example, mg and cl. Elements from opposite sides of the periodic table; Web which elements form ionic bonds? (b) list five examples of compounds with ionic bonds.
Ionic Bond Definition Easy Hard Science
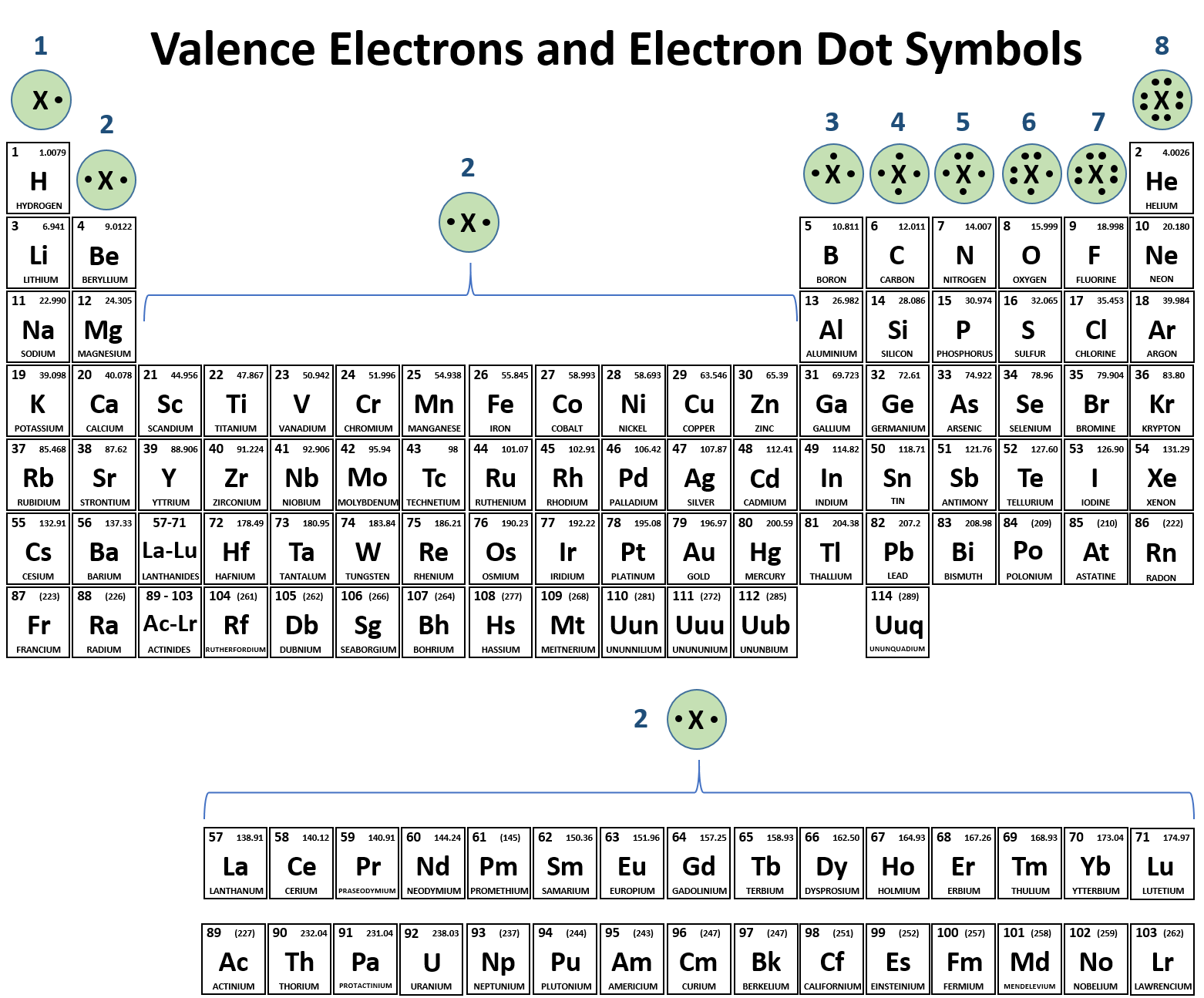
1) for example, consider na and cl. Web ionic bonding is the complete transfer of valence electron(s) between atoms. Web ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. One that tends to lose electrons and one that tends to gain them suppose an oxygen atom gains.
Ionic Bond Definition, Types, Properties & Examples
Web which elements tend to form ionic bonds? Ionic bonds covalent bonds nacl. The atom that loses the electrons becomes a positively charged ion (. (b) list five examples of compounds with ionic bonds. Name at least five ionic bonds and five covalent bonds and enter them in the table below.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
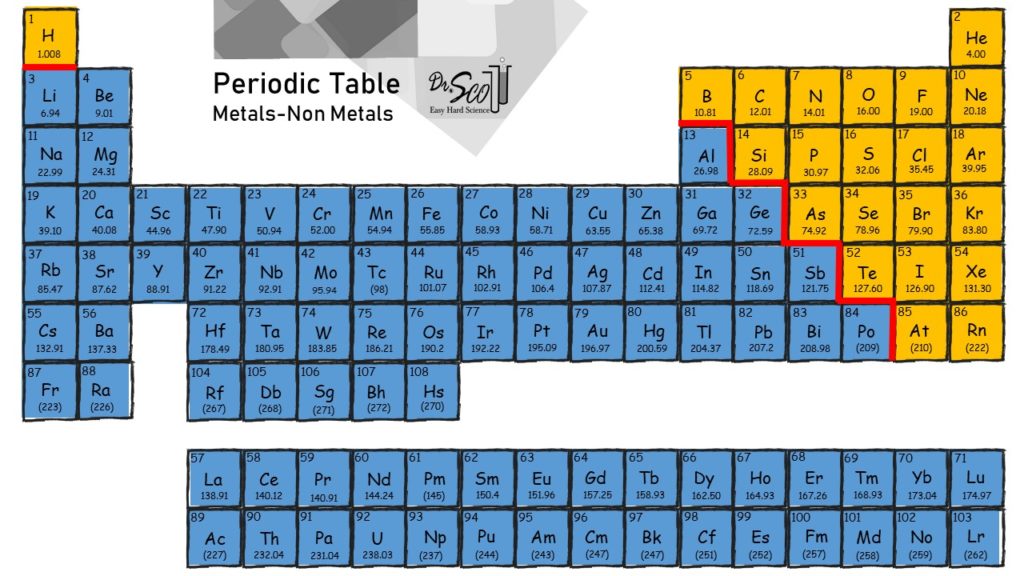
Metals and nonmetals, electrons transferred. Web ionic bonding is the complete transfer of valence electron(s) between atoms. Elements from opposite sides of the periodic table; Web barium (ba) phosphorus (p) radon (rn) lithium (li) In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion.
Examples of Ionic Bonds and Compounds
Web which elements form ionic bonds? Web barium (ba) phosphorus (p) radon (rn) lithium (li) Which of the following elements is likely to form ionic bonds? (b) list five examples of compounds with ionic bonds. Metals and nonmetals, electrons transferred.
Ionic Properties
Which of the following elements is likely to form ionic bonds? Web use the periodic table to describe which elements form ionic bonds and which elements form covalent bonds. 3) last example, mg and cl. Ionic bonds covalent bonds nacl. It is a type of chemical bond that generates two oppositely charged ions.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
The atom that loses the electrons becomes a positively charged ion (. In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion. Web ionic bonding is the complete transfer of valence electron(s) between atoms. Metals and metals, electrons freely moving. Web chemistry chemistry questions and.
Ionic Bond Definition, Types, Properties & Examples
(b) list five examples of compounds with ionic bonds. Web between which type of elements do ionic bonds occur, and how do electrons act within the bond? Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. 2) another example, magnesium and oxygen. Web ionic bond, also called electrovalent bond, type of.
Examples of Ionic Bonding YouTube
1) for example, consider na and cl. Web chemistry chemistry questions and answers (a) use the periodic table to describe which elements form ionic bonds, and which elements form covalent bonds. Web which elements form ionic bonds? (select all that apply.) chlorine (cl) calcium (ca) helium (he) sodium (na) fluorine (f) carbon (c), potassium is a _____. In ionic bonds,.
Metals And Metals, Electrons Freely Moving.
One that tends to lose electrons and one that tends to gain them suppose an oxygen atom gains two electrons to become an oxygen ion. 2) another example, magnesium and oxygen. Web chemistry chemistry questions and answers (a) use the periodic table to describe which elements form ionic bonds, and which elements form covalent bonds. Web between which type of elements do ionic bonds occur, and how do electrons act within the bond?
Web Which Elements Form Ionic Bonds?
3) last example, mg and cl. Web use the periodic table to describe which elements form ionic bonds and which elements form covalent bonds. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. (select all that apply.) chlorine (cl) calcium (ca) helium (he) sodium (na) fluorine (f) carbon (c), potassium is a _____.
(B) List Five Examples Of Compounds With Ionic Bonds.
It is a type of chemical bond that generates two oppositely charged ions. Metals and nonmetals, electrons transferred. Elements from opposite sides of the periodic table; Name at least five ionic bonds and five covalent bonds and enter them in the table below.
One Has Been Shown For You.
1) for example, consider na and cl. The atom that loses the electrons becomes a positively charged ion (. In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion. Web ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound.
.PNG)


/ionic-bond-58fd4ea73df78ca1590682ad.jpg)