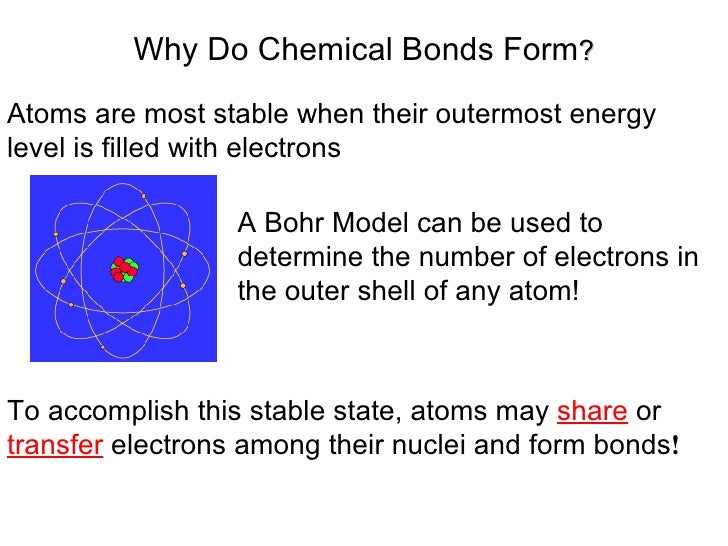
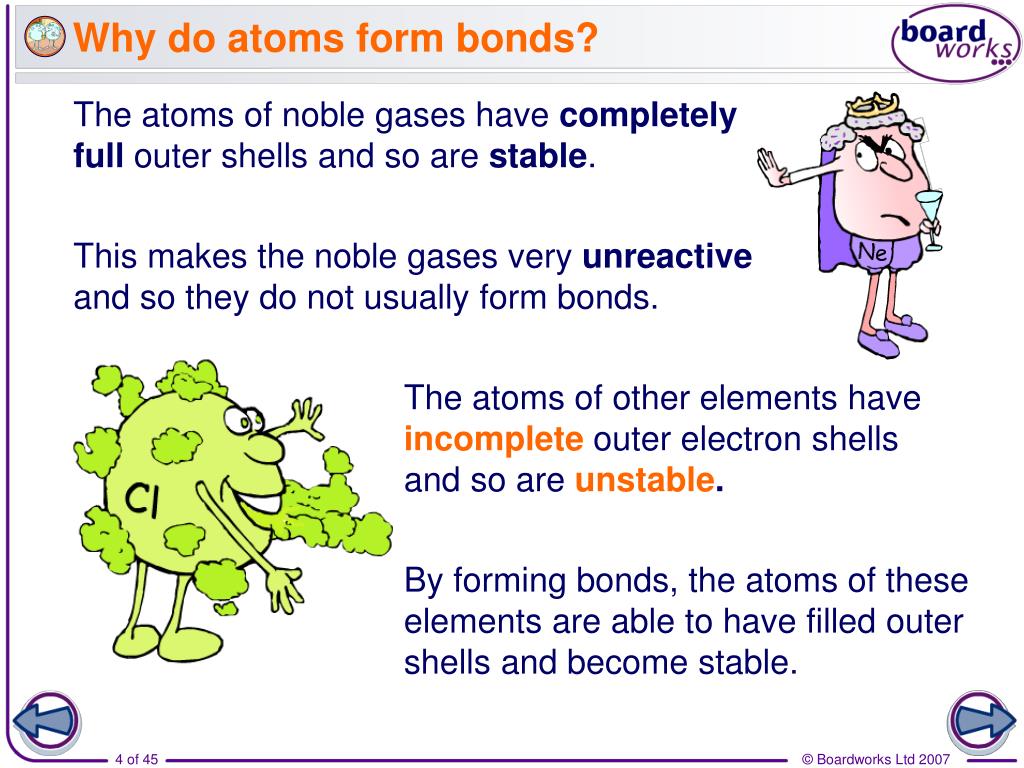
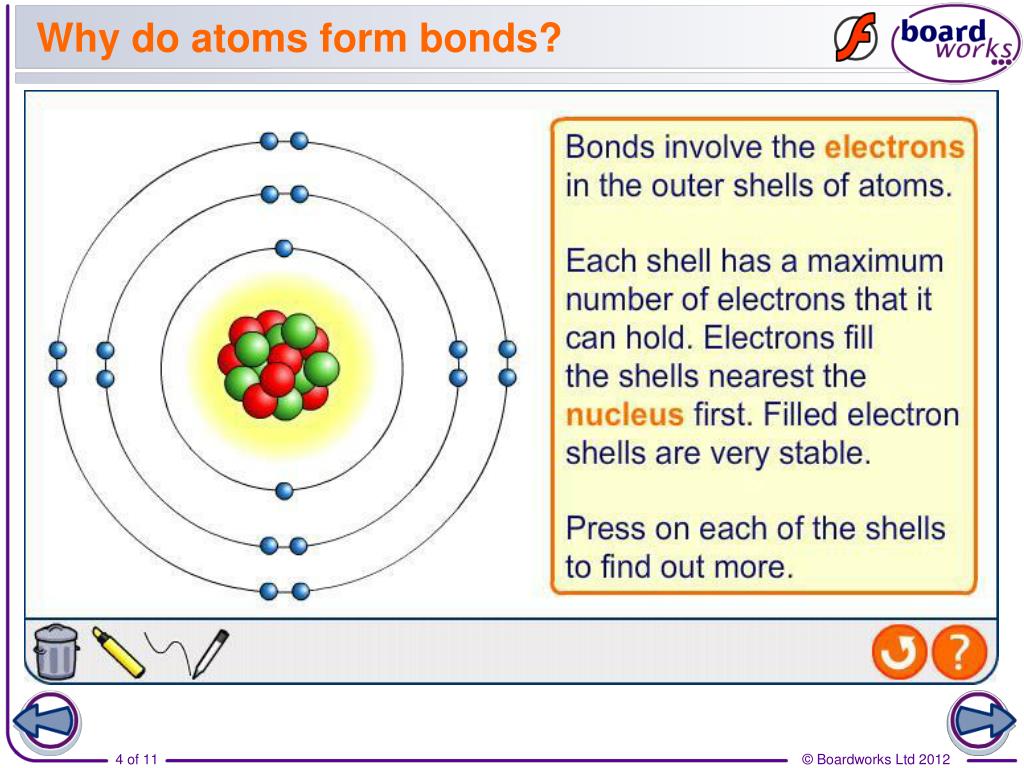
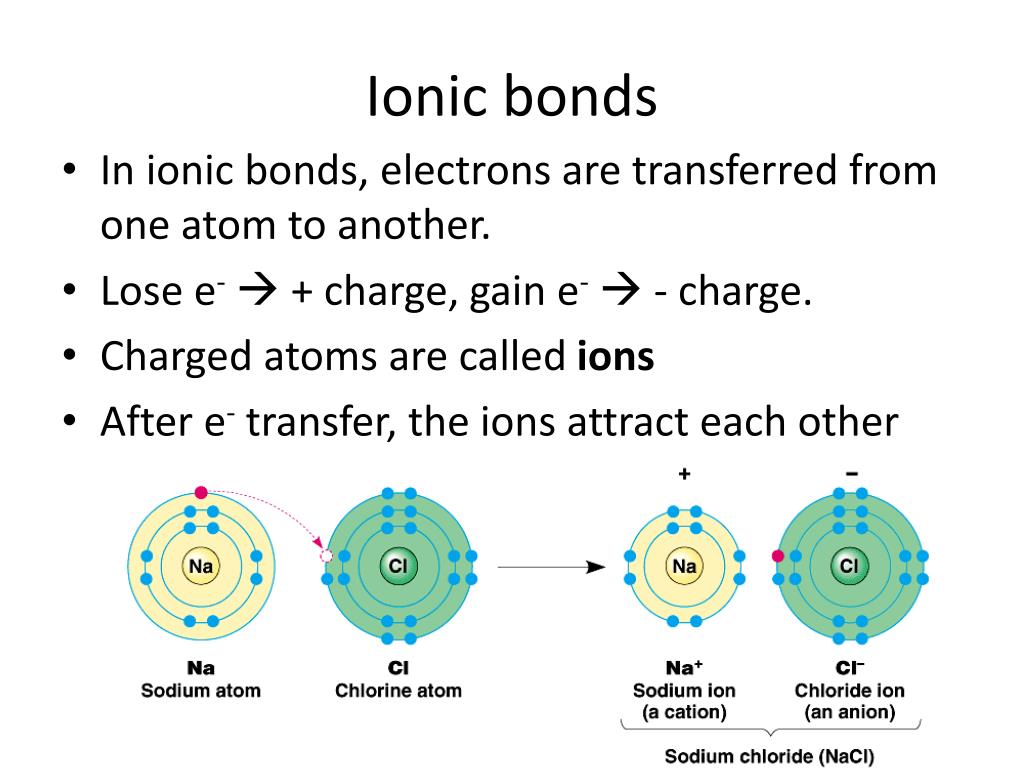
Why Do Atoms Form Chemical Bonds
Why Do Atoms Form Chemical Bonds - Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. Web chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other species. There are three different types of chemical bonds:. Web bonds form when atoms share or transfer valence electrons. Web posted by city chemical on monday, 01 april 2019. Web why do atoms form chemical bonds? Web why form chemical bonds? All bonds appear to link atoms through a sharing of — or an attempt to share — electrons. Attractive forces between atoms that are strong enough to make the linked elements function as a single unit.
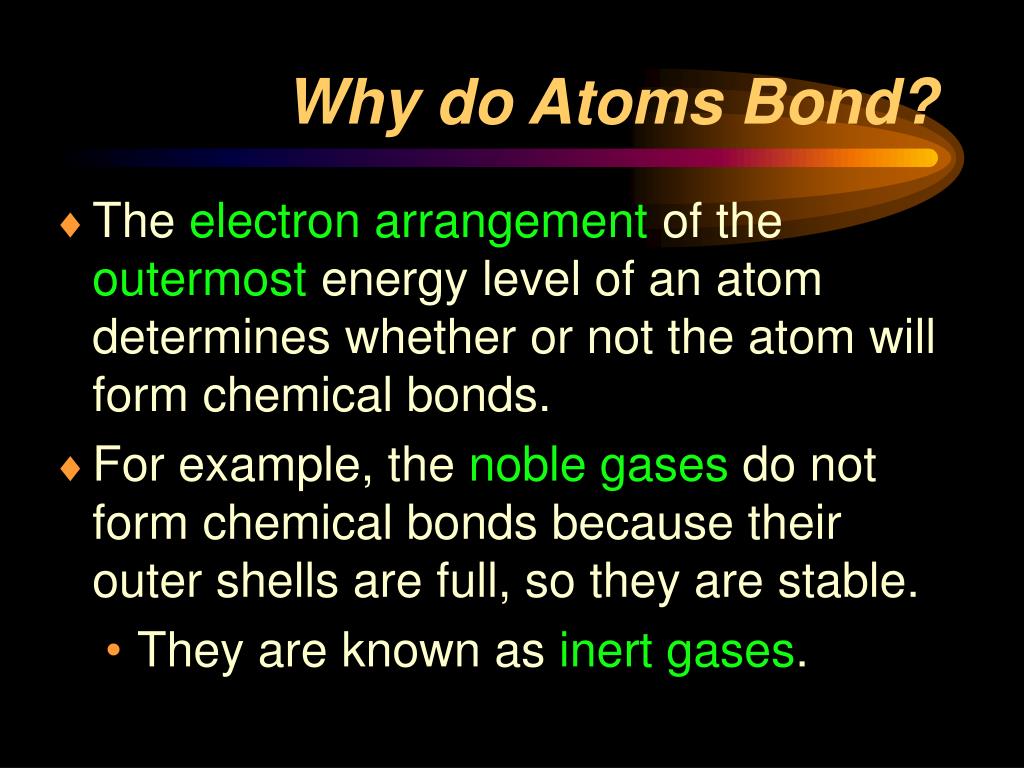
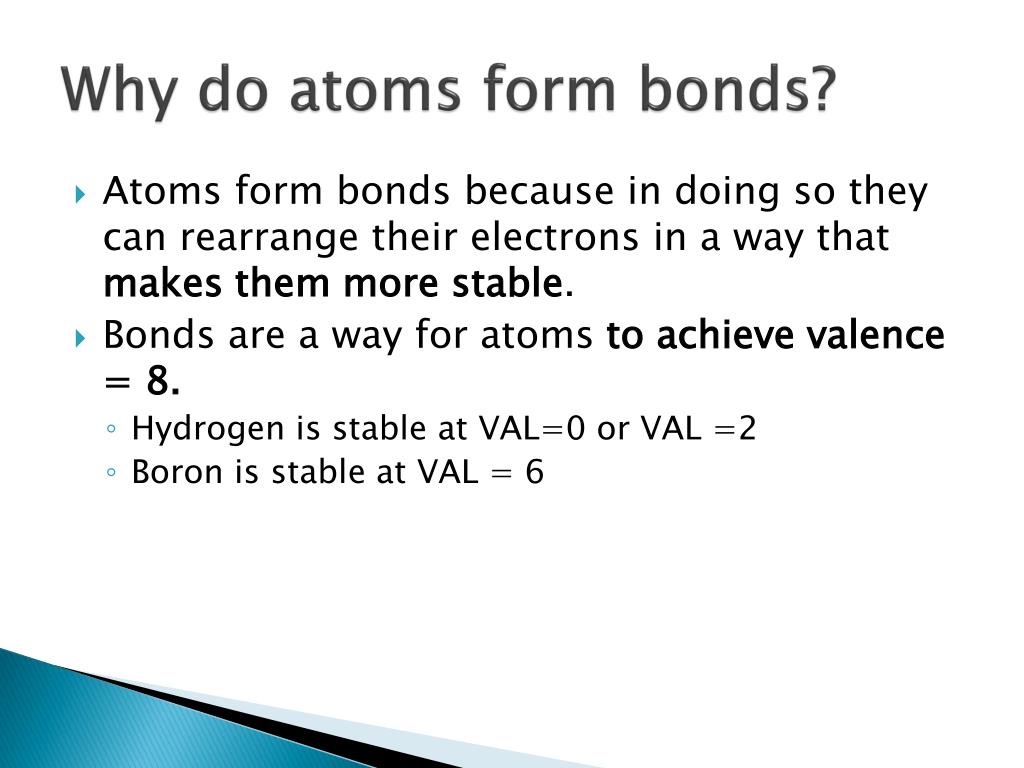
Web chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other species. Atoms form chemical bonds to achieve a full outer energy level, which is the most stable arrangement of electrons. Many atoms become stable when their valence shell is filled with electrons or when they satisfy the. Web bonds form when atoms share or transfer valence electrons. Web why do atoms bond? Web why do atoms form chemical bonds? At the start of this article, we introduced you to a chemical bond: The attraction between different atoms that enables the formation of molecules or compounds. Attractive forces between atoms that are strong enough to make the linked elements function as a single unit. According to the types of bonds contained in a molecule, the physical properties including melting point, hardness, electrical and thermal conductivity and solubility are determined.
(often used as a synonym for chemical) a compound. The type of chemical bond maximizes the stability of the atoms that form it. Some of the attractive forces are weak, some are very strong. Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. Web why do atoms form chemical bonds? Web atoms form chemical bonds to make their outer electron shells more stable. The attraction between different atoms that enables the formation of molecules or compounds. Web chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other species. Web sep 4, 2021 3.2: The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds.
Biochemistry Honors
There are three different types of chemical bonds:. Web chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other species. Web posted by city chemical on monday, 01 april 2019. 149) why does the octet rule not always refer to a stable arrangement of eight valence electrons? Attractive forces between atoms.
Characteristics of Chemical Bonds 1. To understand why atoms form bonds
Atoms form chemical bonds to achieve a full outer energy level, which is the most stable arrangement of electrons. At the start of this article, we introduced you to a chemical bond: Web chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other species. Attractive forces between atoms that are strong.
PPT Why do atoms form bonds? PowerPoint Presentation, free download
The attraction between different atoms that enables the formation of molecules or compounds. Simply put, atoms form bonds in order to become more stable. Web posted by city chemical on monday, 01 april 2019. Atoms form chemical bonds because they want a lower energy and more stable electron configuration. The type of chemical bond maximizes the stability of the atoms.
PPT Ions PowerPoint Presentation, free download ID6738771
Atoms form chemical bonds because they want a lower energy and more stable electron configuration. 149) why does the octet rule not always refer to a stable arrangement of eight valence electrons? Web why form chemical bonds? Atoms form chemical bonds to achieve a full outer energy level, which is the most stable arrangement of electrons. Some of the attractive.
PPT Atomic structure and chemical bonding PowerPoint Presentation
Web bonds form when atoms share or transfer valence electrons. When atoms approach one another, their electrons interact and tend to distribute themselves in space so that the total energy is lower than it would be in any alternative arrangement. All bonds appear to link atoms through a sharing of — or an attempt to share — electrons. According to.
Why Do Atoms Form Chemical Bond in Urdu Hindi Lecture /Chemistry for
When atoms approach one another, their electrons interact and tend to distribute themselves in space so that the total energy is lower than it would be in any alternative arrangement. There are three different types of chemical bonds:. According to the types of bonds contained in a molecule, the physical properties including melting point, hardness, electrical and thermal conductivity and.
PPT Chapter 7 Atoms & Bonding PowerPoint Presentation, free download
Web why do atoms bond? When atoms approach one another, their electrons interact and tend to distribute themselves in space so that the total energy is lower than it would be in any alternative arrangement. Attractive forces between atoms that are strong enough to make the linked elements function as a single unit. Some of the attractive forces are weak,.
CHEMISTRY 9TH CH 4, LECTURE 1, Why do Atoms Form Chemical Bonds
(often used as a synonym for chemical) a compound. Web sep 4, 2021 3.2: At the start of this article, we introduced you to a chemical bond: There are three different types of chemical bonds:. But why do atoms bond to each other in this way?
PPT Chemical Bonding What and Why PowerPoint Presentation, free
Attractive forces between atoms that are strong enough to make the linked elements function as a single unit. Web bonds form when atoms share or transfer valence electrons. Web posted by city chemical on monday, 01 april 2019. Web why do atoms form chemical bonds? There are three different types of chemical bonds:.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
Web bonds form when atoms share or transfer valence electrons. All bonds appear to link atoms through a sharing of — or an attempt to share — electrons. Web why do atoms form chemical bonds? Attraction between atoms or ions leads to a chemical bond. Web why form chemical bonds?
Web Why Do Atoms Form Chemical Bonds?
Attraction between atoms or ions leads to a chemical bond. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. (often used as a synonym for chemical) a compound. When atoms approach one another, their electrons interact and tend to distribute themselves in space so that the total energy is lower than it would be in any alternative arrangement.
The Attraction Between Different Atoms That Enables The Formation Of Molecules Or Compounds.
At the start of this article, we introduced you to a chemical bond: There are three different types of chemical bonds:. Simply put, atoms form bonds in order to become more stable. Web bonds form when atoms share or transfer valence electrons.
According To The Types Of Bonds Contained In A Molecule, The Physical Properties Including Melting Point, Hardness, Electrical And Thermal Conductivity And Solubility Are Determined.
But why do atoms bond to each other in this way? Web why form chemical bonds? Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. Attractive forces between atoms that are strong enough to make the linked elements function as a single unit.
Atoms Form Chemical Bonds Because They Want A Lower Energy And More Stable Electron Configuration.
Web atoms form chemical bonds to make their outer electron shells more stable. Many atoms become stable when their valence shell is filled with electrons or when they satisfy the. Web why do atoms bond? Web chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other species.