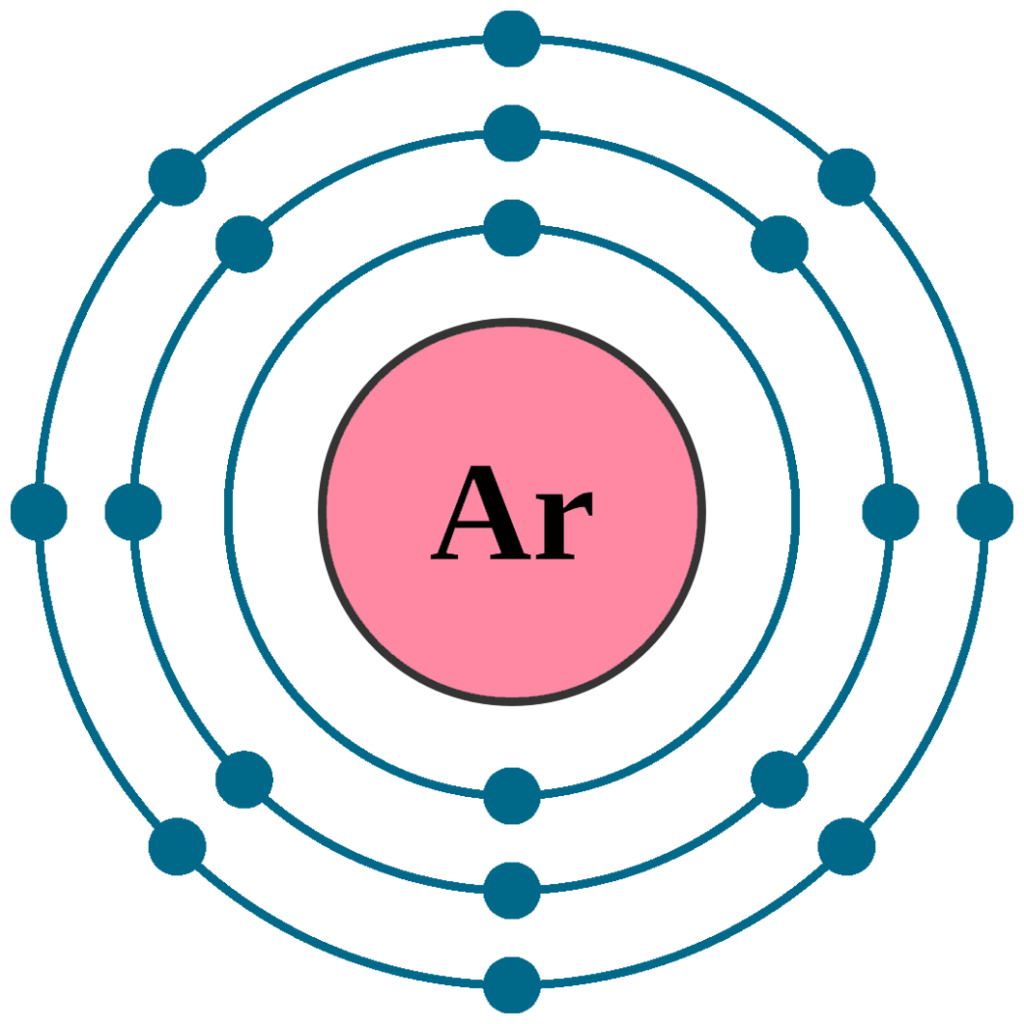
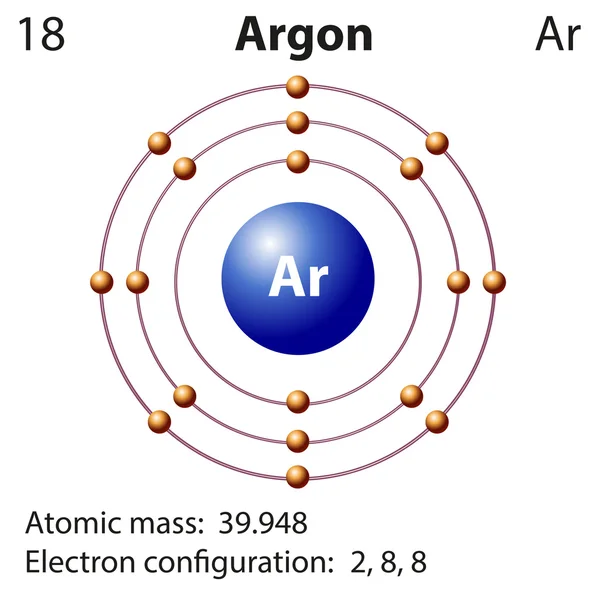
Will Argon Tend To Form Bonds With Other Elements
Will Argon Tend To Form Bonds With Other Elements - Web the atomic number of argon is 18. Web this makes it a noble gas, which are generally unreactive and do not tend to form bonds with other elements. Elements follow what is commonly termed the 8 is great. Web elements usually end up forming a giant metallically bonded lattice if they have a low number of valence electrons. Web the number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. But in the 23 august issue of nature, chemists report that. Web argon's name comes from the greek word argos meaning lazy and indeed for more than a hundred years after its discovery chemists were unable to get it to combine with any. Web biology questions and answers. Will argon tend to form bonds with other elements? Will argon tend to form bonds with other elements?
Unhappy elements formed bonds to create molecules, whereas happy elements remained. Argon atoms would not tend to make bonds with other atoms since it is classified as a noble atom which means it has a complete filled subshells. Will argon tend to form bonds with other elements? Will argon tend to form bonds with other elements? It's one of the inert gases that normally exist as single atoms. Will argon be likely to form bonds with other elements? Of the following elements, the one that is most likely to. The maximum number of electrons that can be held in the orbitals in an atom’s second energy level is 2. Web group 18 elements (helium, neon, and argon) have a full outer, or valence, shell. Web if argon doesn’t form ions to bond with other elements can it even be ionic?
Will argon be likely to form bonds with other elements? Web elements usually end up forming a giant metallically bonded lattice if they have a low number of valence electrons. A clue comes by considering the noble gas elements, the. Of the following elements, the one that is most likely to. The maximum number of electrons that can be held in the orbitals in an atom’s second energy level is 2. Elements in other groups have. Will argon tend to form bonds with other elements? No, it generally does not form chemical bonds because it is a noble. Web it does not form bonds with other elements. Web group 18 elements (helium, neon, and argon) have a full outer, or valence, shell.
Argon form Periodic Table of Elements — Stock Photo © fambros 3096120
Web the atomic number of argon is 18. Web the element argon has always been a loner. It's one of the inert gases that normally exist as single atoms. Web what causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? A full valence shell is the most stable electron configuration.
Argon atoms form cylindrical configurations after collision of two
It's one of the inert gases that normally exist as single atoms. Web the atomic number of argon is 18. Web what causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? Web the number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds.
Why do Noble Gases rarely form Bonds with other Atoms? MakeTheBrainHappy
Web the atomic number of argon is 18. Web this makes it a noble gas, which are generally unreactive and do not tend to form bonds with other elements. Elements in other groups have. Elements follow what is commonly termed the 8 is great. Web what causes atoms to make a chemical bond with other atoms, rather than remaining as.
1 of 18 ng Goal Review Constants I Per... Physical Chemistry
Moving from the far left to the right on the periodic. Web this makes it a noble gas, which are generally unreactive and do not tend to form bonds with other elements. Web what causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? But in the 23 august issue of nature, chemists report.
Argon Ar (Element 18) of Periodic Table Elements FlashCards
Will argon tend to form bonds with other elements? Web biology questions and answers. Please check the table and with the element argon listed, because how can it give/get/share if it. Web argon's name comes from the greek word argos meaning lazy and indeed for more than a hundred years after its discovery chemists were unable to get it to.
Diagram representation of the element neon Stock Vector Image by
Therefore, based on its electron configuration and position on the. But in the 23 august issue of nature, chemists report that. Web it does not form bonds with other elements. The maximum number of electrons that can be held in the orbitals in an atom’s second energy level is 2. Web this makes it a noble gas, which are generally.
Reactivity CLASSWORK Why are argon and neon unlikely to... Math
Web what causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? Will argon tend to form bonds with other elements? Web biology questions and answers. Will argon tend to form bonds with other elements? Unhappy elements formed bonds to create molecules, whereas happy elements remained.
Sample from the RGB Set, a sample of the element Argon in the Periodic
Web what causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? Group 1 element atoms form a metallically bonded lattice. But in the 23 august issue of nature, chemists report that. Elements follow what is commonly termed the 8 is great. A full valence shell is the most stable electron configuration.
Argon Form Periodic Table Of Elements Stock Illustration Illustration
Argon is one of the noble gases (group 18) and thus does not normally combine with other elements. Will argon be likely to form bonds with other elements? Group 1 element atoms form a metallically bonded lattice. Web group 18 elements (helium, neon, and argon) have a full outer, or valence, shell. Will argon tend to form bonds with other.
Reading Covalent Bonds Biology I
Of the following elements, the one that is most likely to. Moving from the far left to the right on the periodic. Therefore, based on its electron configuration and position on the. Argon atoms would not tend to make bonds with other atoms since it is classified as a noble atom which means it has a complete filled subshells. The.
A Full Valence Shell Is The Most Stable Electron Configuration.
Unhappy elements formed bonds to create molecules, whereas happy elements remained. Moving from the far left to the right on the periodic. Elements follow what is commonly termed the 8 is great. It's one of the inert gases that normally exist as single atoms.
Elements In Other Groups Have.
Argon is one of the noble gases (group 18) and thus does not normally combine with other elements. Web argon is element number 18 and has. Web this makes it a noble gas, which are generally unreactive and do not tend to form bonds with other elements. Web group 18 elements (helium, neon, and argon) have a full outer, or valence, shell.
Will Argon Tend To Form Bonds With Other Elements?
Please check the table and with the element argon listed, because how can it give/get/share if it. No, it generally does not form chemical bonds because it is a noble. Web if argon doesn’t form ions to bond with other elements can it even be ionic? Therefore, based on its electron configuration and position on the.
The Maximum Number Of Electrons That Can Be Held In The Orbitals In An Atom’s Second Energy Level Is 2.
Web it does not form bonds with other elements. Web elements usually end up forming a giant metallically bonded lattice if they have a low number of valence electrons. Web the number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. A clue comes by considering the noble gas elements, the.