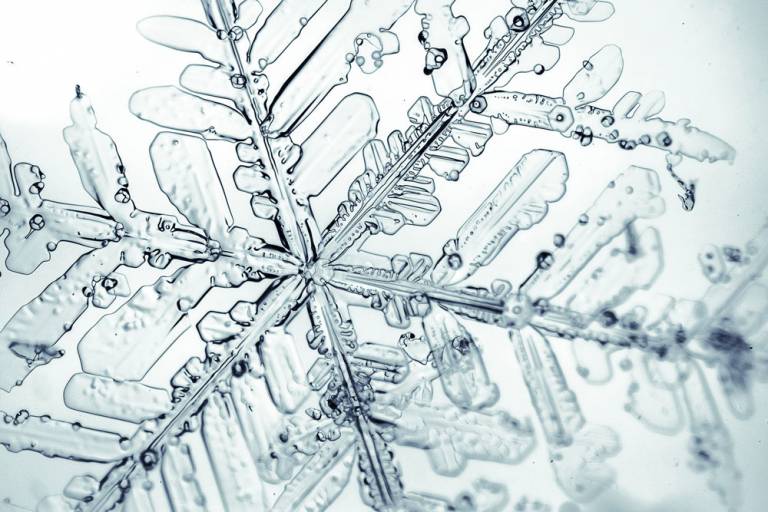
What Do Ice Crystals Form When They Stick Together
What Do Ice Crystals Form When They Stick Together - Though no two snowflakes are exactly. In addition to hydrogen bonding and the. Web this could also cause a chain reaction that produces many ice crystals. The two hydrogen atoms form an angle of 104.5 degrees. Web as they collide, some of them stick together to form larger drops. Web ice crystals are made of water molecules, which are formed by two hydrogen atoms and one oxygen atom. Sometimes, ice crystals and water droplets form together. Web large snowflakes are aggregates of ice crystals. This process of collision and sticking is called. As these ice crystals fall, they can collide and stick together.
Web as they collide, some of them stick together to form larger drops. Web at temperatures below freezing, ice crystals may form around tiny particles of matter, just as water droplets are formed. The two hydrogen atoms form an angle of 104.5 degrees. Web when ice crystals form in water that contains impurities, they are more likely to stick together and form clumps or chunks. Fall speed of ice crystals is important for ice crystal growth by collision and capture. Sometimes, ice crystals and water droplets form together. Web aggregation is the clumping together of ice crystals to form snowflakes. This process of collision and sticking is called. Web large snowflakes are aggregates of ice crystals. In addition to hydrogen bonding and the.
Web when ice crystals form in water that contains impurities, they are more likely to stick together and form clumps or chunks. In addition to hydrogen bonding and the. Web at temperatures below freezing, ice crystals may form around tiny particles of matter, just as water droplets are formed. Web aggregation is the clumping together of ice crystals to form snowflakes. Aggregation is the process by which ice crystals collide and form a single larger ice particle. Web this could also cause a chain reaction that produces many ice crystals. Sometimes, ice crystals and water droplets form together. Web breathe in and my nostrils stick together… yes, it is cold… and snow is falling… but i’ll have to wait for another snowfall to make a snowman! Web ok, so why do we get ice crystals at all, then? Web ice crystals are made of water molecules, which are formed by two hydrogen atoms and one oxygen atom.
Pin on science
Web the study, published today in science and funded by the european research council and royal society, found ice starts to form at exposed surface defects, such as edges,. Web this could also cause a chain reaction that produces many ice crystals. Though no two snowflakes are exactly. Fall speed of ice crystals is important for ice crystal growth by.
Solved J. Analyze these two photomicrographs of ice crysta
Web ok, so why do we get ice crystals at all, then? Web breathe in and my nostrils stick together… yes, it is cold… and snow is falling… but i’ll have to wait for another snowfall to make a snowman! The two hydrogen atoms form an angle of 104.5 degrees. Web when ice crystals form in water that contains impurities,.
Watch ice crystals form on the surface of a bubble as it freezes in
Aggregation is the process by which ice crystals collide and form a single larger ice particle. Though no two snowflakes are exactly. Because, randomly, sometimes a water molecule will condense out right on top of another one. Fall speed of ice crystals is important for ice crystal growth by collision and capture. Web breathe in and my nostrils stick together….
Crystallization and Geodes Discovery Express
Web the study, published today in science and funded by the european research council and royal society, found ice starts to form at exposed surface defects, such as edges,. Aggregation is the process by which ice crystals collide and form a single larger ice particle. Web ice crystals are made of water molecules, which are formed by two hydrogen atoms.
How do ice crystals form and grow? The Weather Guys
This process of collision and sticking is called. Web large snowflakes are aggregates of ice crystals. Aggregation is the process by which ice crystals collide and form a single larger ice particle. Web ok, so why do we get ice crystals at all, then? In addition to hydrogen bonding and the.
Understanding how ice crystals form in clouds UCL News UCL
Web ok, so why do we get ice crystals at all, then? As these ice crystals fall, they can collide and stick together. Web this could also cause a chain reaction that produces many ice crystals. Web as they collide, some of them stick together to form larger drops. Because, randomly, sometimes a water molecule will condense out right on.
What Do Ice Crystals in Meat Signify Meatsliced
Web ok, so why do we get ice crystals at all, then? Because, randomly, sometimes a water molecule will condense out right on top of another one. Web as they collide, some of them stick together to form larger drops. Sometimes, ice crystals and water droplets form together. Aggregation is the process by which ice crystals collide and form a.
How do ice crystals form and grow? The Weather Guys
Web ice crystals are made of water molecules, which are formed by two hydrogen atoms and one oxygen atom. This process of collision and sticking is called. Aggregation is the process by which ice crystals collide and form a single larger ice particle. Web breathe in and my nostrils stick together… yes, it is cold… and snow is falling… but.
What Do ICE Crystals in Meat Signify?
Aggregation is the process by which ice crystals collide and form a single larger ice particle. Web the study, published today in science and funded by the european research council and royal society, found ice starts to form at exposed surface defects, such as edges,. The two hydrogen atoms form an angle of 104.5 degrees. Web this could also cause.
Arts and Crafts How to Make 6Point Snowflakes Presentation Winter
Web large snowflakes are aggregates of ice crystals. Sometimes, ice crystals and water droplets form together. Though no two snowflakes are exactly. Web at temperatures below freezing, ice crystals may form around tiny particles of matter, just as water droplets are formed. Fall speed of ice crystals is important for ice crystal growth by collision and capture.
Because, Randomly, Sometimes A Water Molecule Will Condense Out Right On Top Of Another One.
This process of collision and sticking is called. Web the study, published today in science and funded by the european research council and royal society, found ice starts to form at exposed surface defects, such as edges,. Web solution the correct option is c snowfall when the water droplets in the clouds start freezing into ice crystals and stick together, they will become heavy and eventually fall to the. Web breathe in and my nostrils stick together… yes, it is cold… and snow is falling… but i’ll have to wait for another snowfall to make a snowman!
Web Ice Crystals Are Made Of Water Molecules, Which Are Formed By Two Hydrogen Atoms And One Oxygen Atom.
Sometimes, ice crystals and water droplets form together. Web as they collide, some of them stick together to form larger drops. Fall speed of ice crystals is important for ice crystal growth by collision and capture. In addition to hydrogen bonding and the.
Web When Ice Crystals Form In Water That Contains Impurities, They Are More Likely To Stick Together And Form Clumps Or Chunks.
As these ice crystals fall, they can collide and stick together. Web large snowflakes are aggregates of ice crystals. The two hydrogen atoms form an angle of 104.5 degrees. The larger cloud droplets grow at the expense of smaller ones, and actually become more effective in the.
Aggregation Is The Process By Which Ice Crystals Collide And Form A Single Larger Ice Particle.
Web at temperatures below freezing, ice crystals may form around tiny particles of matter, just as water droplets are formed. Web ok, so why do we get ice crystals at all, then? Though no two snowflakes are exactly. Web this could also cause a chain reaction that produces many ice crystals.